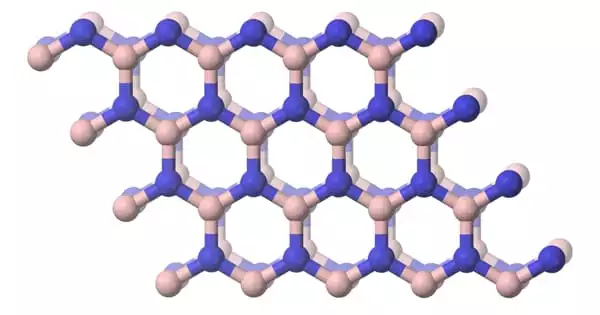
Carbide iodides are anion compounds that combine iodide and carbide anions. It is an ionic compound composed of carbide ions (C²⁻) and iodide ions (I⁻). However, carbide iodides are not well documented in chemistry, and there is little information accessible regarding their specific properties or applications due to their theoretical and less practical significance when compared to other carbide compounds.
Many carbide iodides are cluster compounds, with one, two, or more carbon atoms in the core, surrounded by a layer of metal atoms and enclosed in an iodide ion shell. These ions can be distributed across clusters to form chains, double chains, or layers.
Carbides, such calcium carbide (CaC₂) and silicon carbide (SiC), are often discussed for their industrial applications or chemical reactivity. In comparison, carbide iodide is uncommon and understudied. The creation of carbide iodides may be studied theoretically or in specific research contexts, but it is not a commonly recognized or used compound in practical applications.
The metal in carbide iodides is typically a rare earth element. Similar formulas typically have similar structures. R12C6I17 is a rare earth element composed of R6 octahedra with a C26− core and an iodide shell. R4I5C has similar chains, but with a single C4− carbide atom. R6I7C2 and R3I3C are two examples of double chain architectures with a single carbon atom core. Layers of connected octahedra include R2I2C2 with an ethanide C24− core, as well as R2I2C and R2IC, each containing one carbide.
Related compounds include carbide chlorides and carbide bromides. In cluster compounds, carbon can be replaced with hydrogen, boron, or nitrogen. This list excludes cyanides, carbonyls, cyanamides, and carbido borates, which are nonmetal compounds with carbon bonds. However, there are carbide iodides that also contain nitride, oxide or other halides.
Some examples of carbide iodides include:
- Calcium Carbide Iodide (CaC2I2): This compound forms when calcium carbide (CaC2) reacts with iodine. It is a white to off-white solid and is used in various chemical reactions.
- Barium Carbide Iodide (BaC2I2): Similar to calcium carbide iodide, this compound is formed by the reaction between barium carbide and iodine.
- Strontium Carbide Iodide (SrC2I2): Another example where strontium carbide reacts with iodine to produce the carbide iodide.
These compounds are mainly of interest in chemical research and synthesis, rather than in practical applications, due to their specific chemical properties and reactivity. They are part of the broader family of carbide salts where the carbide ion is bonded to a metal cation and can participate in various chemical reactions.