Calcium monophosphide is the inorganic compound with the formula CaP. It is an inorganic compound composed of calcium and phosphorus. It is sometimes also known as “calcium phosphide”, which also describes a different compound with composition Ca3P2. Calcium monophosphide is a black solid. It is typically synthesized by direct combination of elemental calcium and phosphorus at high temperatures.
Properties
- Chemical formula: CaP (Ca2P2)
- Appearance: black solid
- Solubility in water: decomposes
- Molar mass: 78.05 g/mol
- Crystal structure: Rock salt (NaCl-type) structure
- Melting point: ~1,600 °C (approximate)
- Density: ~2.89 g/cm³
- Solubility: Insoluble in water; reacts slowly with water forming phosphine (PH₃)
- Reactivity: Reacts with acids and water; releases toxic gases like phosphine
- Magnetic properties: Diamagnetic or weakly paramagnetic depending on form
- Stability: Stable under dry, inert conditions; decomposes in moist air
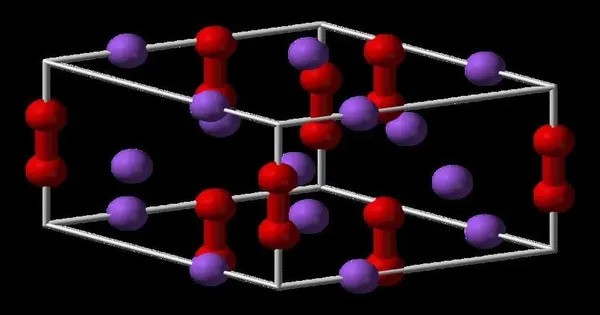
Structure
The structures of CaP and sodium peroxide (Na2O2) are very similar. The solid is described as a salt: (Ca2+)2P24−, or Ca2P2. Since the bonding is ionic, the diphosphide centers carry negative charge and are easily protonated. Upon hydrolysis this material releases diphosphine (P2H4):
Ca2P2 + 4 H2O → 2 Ca(OH)2 + P2H4
The hydrolyses of CaP and calcium carbide (CaC2) are similar, except that diphosphine spontaneously ignites in air. Thus, CaP must be protected from air.
CaP decomposes to Ca3P2 at about 600 °C.
3 CaP → Ca3P2 + 1/4 P4
Chemical Behavior
Reaction with Water:
𝐶𝑎𝑃 + 𝐻2𝑂 → 𝐶𝑎(𝑂𝐻)2 + 𝑃𝐻3↑
This reaction releases phosphine gas, which is toxic and flammable.
Reaction with Acids:
Produces phosphine and corresponding calcium salts.
Occurrence and Production
Natural Occurrence
- Does not occur naturally in free form.
- Calcium and phosphorus naturally occur in other forms, mainly:
- Calcium in limestone (CaCO₃)
- Phosphorus in phosphate rocks (Ca₃(PO₄)₂)
Synthesis (Industrial/ Laboratory)
High-temperature reaction between calcium metal and red phosphorus:
Ca+P → CaP
Usually done under an inert atmosphere (argon or nitrogen) to avoid oxidation or unwanted side reactions.
Safety Considerations
- Phosphine gas released during hydrolysis is toxic, pyrophoric, and can cause respiratory distress.
- CaP should be handled in a dry, inert atmosphere (like a glovebox).
- Proper PPE (gloves, goggles, fume hood) is essential during handling.