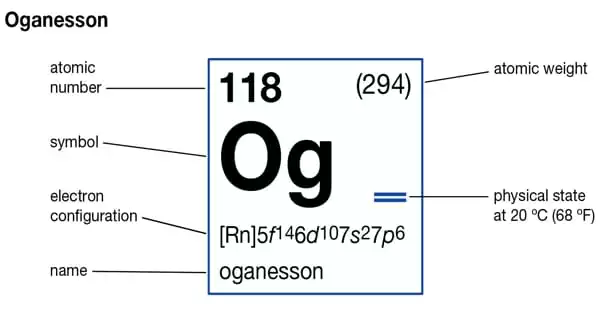
Oganesson is a synthetic chemical element with the symbol Og and the atomic number 118. It is a radioactive element that was created intentionally and about which little is known. It is believed to be a gas and is classified as a non-metal. It belongs to the noble gas group.
It was initially synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a team of Russian and American scientists. It was acknowledged as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP in December 2015. It was formally named on November 28, 2016.
The name honors nuclear physicist Yuri Oganessian, who was instrumental in the discovery of the periodic table’s heaviest elements. It is one of only two elements named after living people at the time of its discovery, the other being Seaborgium, and the only element whose eponym is still alive today.
Properties
Oganesson has a single known isotope, 294Og, with a half-life of around 0.89 milliseconds. It degrades to 290Lv by alpha decay (livermorium-290). The atomic weight of man-made transuranium elements is determined by the isotope with the longest half-life. These atomic weights should be regarded as provisional, as a new isotope with a longer half-life may be created in the future.
- Symbol: Og
- Atomic Number: 118
- Atomic Weight: [294]
- Phase: probably a gas

Oganesson has not been generated in sufficient amounts to be extensively tested. All predictions about its physical and chemical properties are based on theoretical calculations and computational simulations. In contrast to other noble gases, speculations claim that Oganesson would be liquid at ambient temperature and have a boiling point of roughly 320 K – 380 K. Another study suggests that it could be a semiconductor.
Element Classification
Oganesson has the highest atomic number and mass of any known element. The radioactive oganesson atom is extremely unstable, and only five (perhaps six) atoms of the isotope oganesson-294 have been found since 2005. Although this limited experimental characterization of its properties and potential compounds, theoretical calculations resulted in many predictions, some of which were surprising.
For example, oganesson, despite being a member of group 18 (the noble gases) and the first synthetic element to be so, may be extremely reactive, unlike all other elements in that group. It was formerly considered to be a gas under normal conditions, but due to relativistic effects, it is now projected to be solid. It is a p-block element and the final of period 7 on the periodic table of elements.
Discovery
Researchers at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, claimed on October 9, 2006, that they had indirectly found ununoctium-294 from collisions of californium-249 atoms with calcium-48 ions. The first tests that resulted in element 118 were carried out in 2002.
Oganesson does not occur naturally, and it has not been discovered in the earth’s crust, so there is no cause to be concerned about its health risks.