Cadmium iodide (CdI2) is a chemical compound that combines the elements cadmium and iodine. It stands out because of its crystal structure, which is characteristic of MX2 compounds with heavy polarization effects. It is a green-yellow solid that is water, alcohol, ether, acetone, ammonia, and acid-soluble. It’s used in lithography, printing, electroplating, process engraving, analytical chemistry, cadium electrodeposition, and the production of phosphor and lubricants, among other things.
Cadmium iodide is made by reacting cadmium metal, or its oxide, with hydroxide or carbonate, and hydroiodic acid. It’s also possible to make the compound by heating cadmium with iodine. It’s white hexagonal flakes or crystals that slowly turn yellow when exposed to air or light; it comes in two allotropic forms, alpha and beta; density 5.67 g/cm3; melts at 387°C (alpha form) and 404°C (beta form); vaporizes at 742°C; vapor pressures 1 and 5 torr at 416 and 481°C, respectively; soluble in water (86 g/100 mL at 25°C), ethanol, acetone, ether, and ammonia.
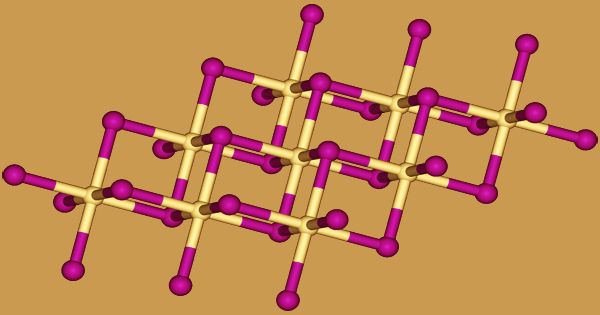
The iodide anions form a hexagonal close packed structure in cadmium iodide, while the cadmium cations alternately fill all of the octahedral sites. The layered lattice that results is the final structure. Many other salts and minerals have the same basic structure. Cadmium iodide is mostly ionically bound, but it also has certain covalent properties. Cadmium iodide is made by mixing hydriodic acid with cadmium metal, or its oxide, hydroxide, nitrate, or carbonate:
CdO + 2HI → CdI2 + H2O
Also, the compound can be made by heating cadmium with iodine:
Cd +I2 → CdI2
Slow crystallization from solutions or fused salt mixtures may yield a brownish crystalline-form of the salt. The crystal structure of cadmium iodide serves as a model for the crystal structures of a variety of other compounds. Compounds with some of the characteristics below are more likely to adopt the CdI2 structure:
- Moderately polarising cations’ iodides; firmly polarising cations’ bromides and chlorides
- Hydroxides of dications, i.e., compounds with the general formula M(OH)2
- Tetracation sulfides, selenides, and tellurides (chalcogenides), i.e., compounds with the general formula MX2, where X = S, Se, and Te.
Cadmium iodide is used in photography, analytical chemistry, phosphor manufacturing, and lubricant manufacturing. As a nematocide, in the manufacture of phosphors, as a lubricant, in photoconductors, in printing, process engraving, lithography, and analytical chemistry. Crystallization of cadmium oxide caused by the action of hydriodic acid. Water, alcohol, and ether are all soluble in the colorless, flaky crystals. For collodion formulas, this halide was a popular iodizer, particularly for negatives.
Information Sources: