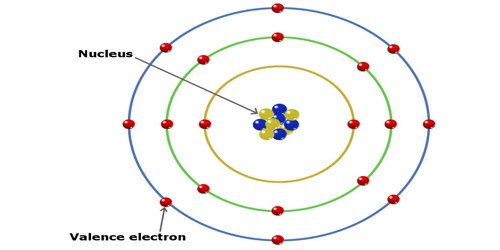
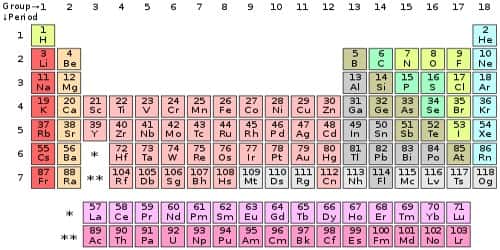
In chemistry, valence electrons are the electrons in the outside or valence electron shell of an atom. They are the electrons located at the outermost shell of an atom. They determine the valency of the atom which is important in how a chemical element reacts with other elements. They are generally the electrons that are farthest from the nucleus. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized.
The valence electrons are the electrons that determine the most typical bonding patterns for an element. They are the ones involved in forming bonds to adjacent atoms. Elements that have eight valence electrons (noble gases) are inert and they do not tend to create chemical reactions with other elements in the Periodic table group. These electrons are found in the s and p orbitals of the highest energy level for the element. For example, Sodium has 1 valence electron from the 3s orbital.
The number of valence electrons in an atom is reflected by its position in the periodic table of the elements. The number of valence electrons is also important for determining the group of an element in the Periodic table because an element’s number of valence electrons is the same as the number of its group in the periodic table. Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. To calculate the number of valence electrons present in an element you have to find the last number of the electron configuration.