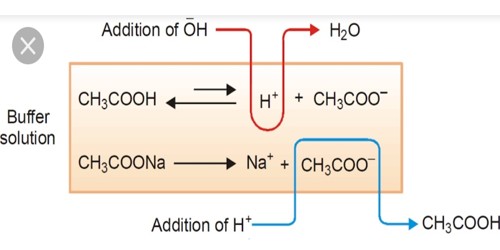
A buffer solution is a chemical solution that resists change to its pH or acidity. It is one that resists changes in pH when small quantities of an acid or an alkali are added to it. It is a solution in water of a mixture of a weak acid or base and its salt. The pH of the solution changes very little when a small amount of strong acid or base is added to it. It only means that the change in pH is not as much as it would be with a solution that is not a buffer.
Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. A lot of biological and chemical reactions need a constant pH for the reaction to proceed. Many life forms have a relatively small pH range; an example of a buffer solution is blood. Buffers are extremely useful in these systems to maintain the pH at a constant value. They are necessary for biology for keeping the correct pH for proteins to work.
Buffer solutions may be of two types: acidic and basic.
- Acidic: A solution of a weak acid and its salt. Acidic buffer solutions are commonly made from a weak acid and one of its salts – often a sodium salt.
- Basic: A solution of a weak base and its salt. An alkaline buffer solution has a pH greater than 7. A frequently used example is a mixture of ammonia solution and ammonium chloride solution.
If you add acid to the solution, the concentration of H+ ions will increase; to keep equilibrium a small number of ions will be combined (forming a salt and reducing the concentration of H+ ion in the solution). The use of one or the other will simply depend upon the desired pH when preparing the buffer. If you add base the concentration of H+ ion will reduce (by consumption or combining) and so a small amount of salt will break into ions and maintain the pH.