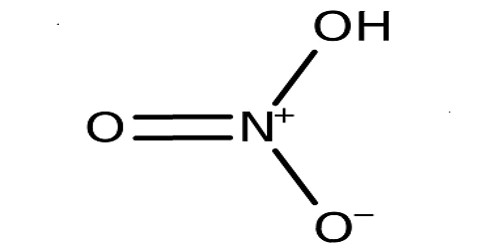
Nitric Acid (HNO3) is a very corrosive and toxic strong acid that can cause severe burns. It is both extremely corrosive and toxic. It is also known as aqua fortis. Nitric Acid is used in rocket fuels, to help make wood look older, and is used in explosives. It is also known as the spirit of niter, which is a highly corrosive mineral acid. Upon distillation, nitric acid in its pure form begins to boil at 78.2°C and becomes solid when it is well cooled. It dissolves metals such as iron, copper, and silver.
It can react with metals such as copper to produce a brown toxic gas called nitrogen dioxide. It is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. It is made by the reaction of nitrogen dioxide with water. It is also commonly used as a strong oxidizing agent. It reacts with metals, oxides, and hydroxides, forming nitrate salts. It is normally considered to be a strong acid at ambient temperatures. It is produced chiefly by oxidation of ammonia through the Ostwald process.
In alchemy, it is known as aqua fortis or strong water. It can dissolve silver but leaves gold unharmed, making it a good test for gold. The term acid test came from this test. It is commonly used in science laboratories at schools for experimenting when specifically testing for chloride. The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. It is a highly corrosive acidic substance.
Uses
- Nitric acid is used in the production of ammonium nitrate for fertilizers, making plastics, and in the manufacture of dyes. It is used in the production of ammonium nitrate and calcium ammonium nitrate which find applications as fertilizers.
- It is used for making explosives such as nitroglycerin and TNT. When it is combined with hydrochloric acid, an element called aqua regia is formed.
- It is used in a colorimetric test to distinguish heroin and morphine.
- In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes.
- It can be used as a spot test for alkaloids like LSD, producing a variety of colors, depending on the alkaloid.
Health Hazards
Nitric acid is an extremely corrosive acid capable of causing severe chemical burns very rapidly. This mineral acid has a variety of applications and presents several health hazards if used without the necessary safety precautions.