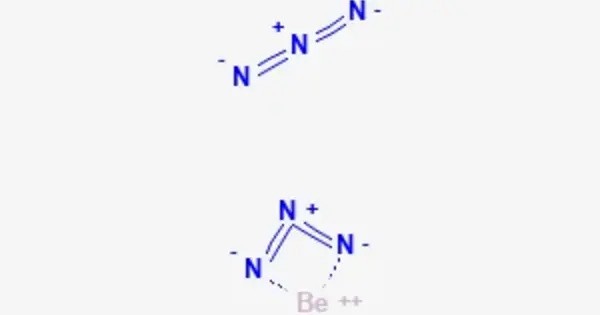
Beryllium azide, Be(N3)2, is an inorganic compound. It is the beryllium analog of hydrazoic acid (HN3). is highly reactive and potentially dangerous due to the instability of the azide ion, which can decompose explosively under certain conditions. It can be soluble in polar solvents like water, but the solubility and stability can depend on the specific conditions.
Beryllium azide is a highly reactive and dangerous compound. Its use is mainly confined to specific industrial or laboratory environments where controlled reactions are necessary, and its synthesis requires strict safety protocols due to the compound’s explosive nature.
Properties
- Chemical formula: Be(N3)2
- Molar mass: 93.054 g·mol−1
- Appearance: white solid
- Solubility: It is soluble in water, though it is generally unstable in aqueous solutions.
- Stability: It is highly unstable and sensitive to mechanical shock, heat, and friction. It is considered a highly explosive compound. Its decomposition can produce toxic gases, including nitrogen oxides.
- Melting Point: The exact melting point is not well documented due to its instability.
- Density: The density is not widely available in standard references, but due to the low molecular weight and high reactivity, it can be inferred to be lower than many other azide compounds.
Synthesis
Beryllium azide has been synthesised by the reaction of beryllium chloride with neat trimethylsilyl azide:
BeCl2 + 2 Me3SiN3 → Be(N3)2 + 2 Me3SiCl
Alternatively, dimethylberyllium reacts with hydrazoic acid in dry diethyl ether at −116 °C:
Be(CH3)2 + 2 HN3 → Be(N3)2 + 2 CH4
Structure
Infrared and Raman spectra suggest that beryllium azide consists of infinite chains, with tetrahedrally coordinated beryllium(II) atoms covalently bridged by one end of the azide units.
Occurrence of Beryllium Azide:
- Natural Occurrence: Beryllium azide does not occur naturally in the environment in any significant quantity. It is typically synthesized for research or industrial purposes, especially in the context of explosives or in the study of azide chemistry.
- Synthesis: Beryllium azide can be synthesized by reacting sodium azide (NaN₃) with a beryllium salt, such as beryllium nitrate (Be(NO₃)₂). The reaction forms beryllium azide and sodium nitrate as a byproduct.
Be(NO3)2+2NaN3→Be(N3)2+2NaNO3
Safety Considerations:
Due to its instability and the fact that azide compounds are highly toxic and potentially explosive, beryllium azide should only be handled by experienced professionals in controlled laboratory environments. The presence of beryllium compounds also introduces health risks such as chronic beryllium disease, which can affect the lungs.
In general, azide compounds are handled with great care because of their tendency to decompose violently under stress, release nitrogen gas, and possibly lead to fire or explosion. The specific risk posed by beryllium azide is magnified by its combination with beryllium, a toxic metal.