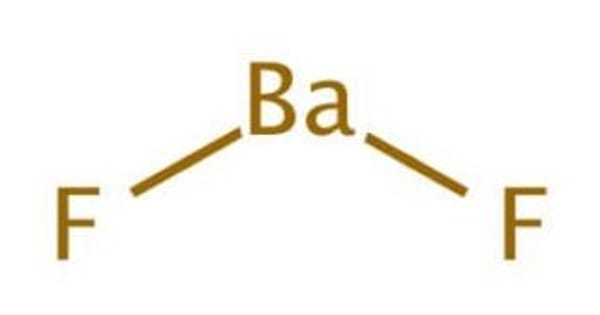
The inorganic compound barium fluoride (BaF2) has the formula BaF2. It exists in nature as the rare mineral frankdicksonite, which is a colorless solid. It is a white, solid, crystalline compound BaF2 that is used in optics (such as lenses) and as a flux in some enamels. It has a fluorite structure under normal conditions and a PbCl2 structure under high pressure. It, like CaF2, is resistant to water and insoluble in it.
The chemical symbol for the metal barium is Ba, and the chemical symbol for the nonmetal fluorine is F. This substance is known as barium fluoride.
Properties
Moisture corrodes BaF2 above about 500°C, but in dry conditions, it can be used up to 800°C. Prolonged moisture exposure reduces transmission in the vacuum UV range. It is less resistant to water than calcium fluoride, but it is the most resistant to high-energy radiation of all the optical fluorides, despite having a lower far-ultraviolet transmittance than the other fluorides. It is quite hard, very sensitive to thermal shock, and easily fractures.
- Molecular Weight: 175.32
- Appearance: white cubic crystals
- Melting Point: 1370 °C (2500 °F)
- Boiling Point: 2260° C
- Density: 4.9 g/cm3
- Morphology: Cubic
- Solubility in H2O: 0.17 g/100 cm3

Optical properties
From 150–200 nm to 11–11.5 m, barium fluoride is transparent from the ultraviolet to the infrared. It is used in windows for infrared spectroscopy, particularly in fuel oil analysis. Its transmittance at 200 nm is relatively low (0.60), but at 500 nm it rises to 0.96–0.97 and remains there until 9 m, when it begins to fall (0.85 for 10 m and 0.42 for 12 m). From 700 nm to 5 m, the refractive index is approximately 1.46.
Gas-phase structure
The BaF2 molecule is non-linear in the vapor phase, with an F-Ba-F angle of about 108°. Its nonlinearity contradicts VSEPR theory. According to ab initio calculations, contributions from d orbitals in the shell below the valence shell are to blame. Another theory is that polarisation of the electron core of the barium atom results in a roughly tetrahedral charge distribution that interacts with the Ba-F bonds.
Application
Barium fluoride is used in the production of welding agents, enamel and glazing frits, and aluminum metallurgy. It is used as a preopacifying agent as well as in the manufacture of enamel and glazing frits. It is also used in the manufacture of welding agents. It is also used as a molten bath for refining aluminum in metallurgy.
It can be found in spectroscopic components. It is frequently used as a viewport window for thermography and is often suitable for applications in the passive IR band (8 to 14 m). Transmission extends approximately 1 micron further into the IR than Calcium Fluoride for an equivalent thickness.