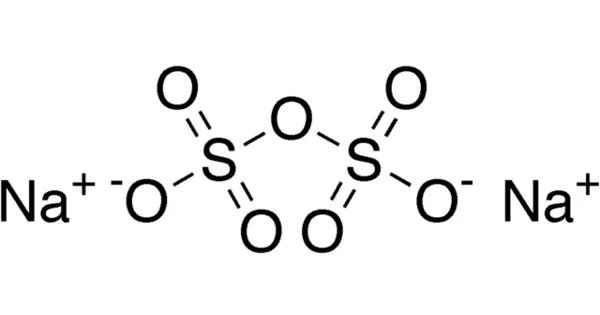
Sodium pyrosulfate has the chemical formula Na2S2O7 and is an inorganic compound. It is a white salt. It has the appearance of a white crystalline solid. It is highly soluble in water and has an acidic aqueous solution. Because the compound is hygroscopic, it readily absorbs moisture from the air.
The primary application of sodium pyrosulfate is as an intermediate in the production of other chemicals. It is widely used in the production of dyes, pigments, and pharmaceuticals. It can also be used to produce sulfuric acid.
Properties
- Chemical formula Na2S2O7
- Molar mass: 222.12 g/mol
- Appearance: Translucent white crystals
- Density: 2.658 g/cm3
- Melting point: 400.9 °C (753.6 °F; 674.0 K)
- Boiling point: decomposes at 460 °C (860 °F; 733 K)
- Solubility in water: hydrolyses
Chemical properties
- Acidic Nature: It is an acidic compound and can act as a source of sulfuric acid when dissolved in water. It can release two moles of sulfuric acid upon hydrolysis.
- Stability: It is relatively stable under normal conditions, but it can decompose at high temperatures, releasing sulfur trioxide (SO3) and oxygen.
- Oxidizing Agent: It has oxidizing properties, and it can oxidize various substances when heated or in the presence of other reducing agents.
- Reaction with Metals: It can react with some metals, such as aluminum and iron, releasing hydrogen gas and forming sulfates.
Preparation
Sodium pyrosulfate is obtained by the dehydration of sodium bisulfate:
2 NaHSO4 → Na2S2O7 + H2O
Temperatures above 460 °C further decompose the compound, producing sodium sulfate and sulfur trioxide:
Na2S2O7 → Na2SO4 + SO3
Applications
In analytical chemistry, sodium pyrosulfate was used. Before quantitative analysis, samples are fused with sodium pyrosulfate to ensure complete dissolution. It is used in water treatment processes to reduce chlorine levels in swimming pools and spas. It can assist in the neutralization of excess chlorine, which can be harmful to the skin and eyes.
Because of its acidity, sodium pyrosulfate is used in some cleaning products. It can aid in the removal of mineral deposits, stains, and scales from a variety of surfaces. It is used as a reagent in laboratories for a variety of chemical reactions and analyses.
Safety
Sodium pyrosulfate should be handled with care as it can be irritant to the skin, eyes, and respiratory system. It is advisable to wear appropriate protective equipment, such as gloves and goggles when working with this compound. Additionally, it should be stored in a cool, dry place away from incompatible substances.