ANP: Atrial Natriuretic Peptide
Definition
Atrial Natriuretic Peptide or ANP is a powerful vasodilator, and a protein (polypeptide) hormone secreted by heart muscle cells. It is also called Atrial Natriuretic Factor (ANF), Atrial Natriuretic Hormone (ANH), Cardionatrine, Cardiodilatin (CDD), or Atriopeptin.
ANP is a peptide hormone, produced by certain cells in the wall of the atrium of the heart that promotes the excretion of sodium ions in the urine. Secretion of ANP is triggered by increased stretch of the atrial wall, due to raised blood pressure or increased blood volume. It acts to inhibit sodium reabsorption in the kidneys and the secretion of aldosterone by the adrenal glands. Consequently, sodium losses to urine are increased, and water follows by osmosis, thereby decreasing blood volume and blood pressure.
ANP was discovered in the early 1980s. de Bold and colleagues in Kingston, Ontario, Canada found that rat atrial extracts contained a substance that increased salt and urine output in the kidney. Later, the substance was purified from the heart by several groups and named ANF or ANP.
Structure and Function of ANP
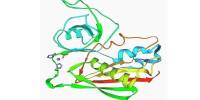
The crystal structures of the dimerized ANP receptor extracellular domain (ECD) with and without ANP have revealed a novel hormone-induced rotation mechanism occurring in the juxtamembrane region that appears to mediate signal transduction. Atrial natriuretic peptide (ANP) also plays a major role in blood pressure and volume regulation. ANP activities are mediated by a cell surface, single-span transmembrane receptor linked to its intrinsic guanylate cyclase activity.
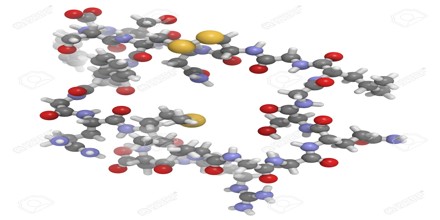
ANP is a 28-amino acid peptide with a 17-amino acid ring in the middle of the molecule. The ring is formed by a disulfide bond between two cysteine residues at positions 7 and 23. ANP is closely related to BNP (brain natriuretic peptide) and CNP (C-type natriuretic peptide), which all share a similar amino acid ring structure.
The ECD crystal packing contains two major intermolecular contacts that suggest two possible dimer pairs: ‘head-to-head’ (hh) and ‘tail-to-tail’ (tt) dimers associated via the membrane-distal and membrane-proximal subdomains, respectively. The existence of these two potential dimer forms challenges the proposed signaling mechanism. In this study, we performed single-particle electron microscopy (EM) to determine the ECD dimer structures occurring in the absence of crystal contacts. EM reconstruction yielded the dimer structures with and without ANP in only the hh dimer forms.
ANP binding caused a time-dependent decrease in Trp emission at 350 nm that was attributable to partially buried Trp74 in the unbound hh dimer interface becoming exposed to solvent water upon ANP binding. Thus, the results of single-particle EM and Trp fluorescence studies have provided direct evidence for hh dimer structures for unbound and ANP-bound receptor. The results also support the proposed rotation mechanism for transmembrane signaling by the ANP receptor.
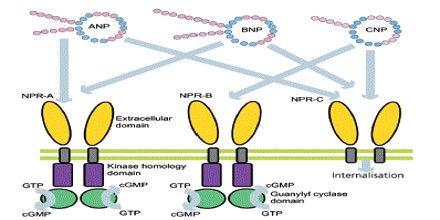
Receptors and Effects of ANP
Atrial Natriuretic Peptide (ANP) receptors (provisional nomenclature) are a family of homodimeric, catalytic receptors with a single TM domain and guanylyl cyclase activity on the intracellular domain of the protein sequence.
Three types of atrial natriuretic peptide receptors have been identified on which natriuretic peptides act. They are all cell surface receptors and designated:
- guanylyl cyclase-A (GC-A) also known as natriuretic peptide receptor-A (NPRA/ANPA) or NPR1
- guanylyl cyclase-B (GC-B) also known as natriuretic peptide receptor-B (NPRB/ANPB) or NPR2
- natriuretic peptide clearance receptor (NPRC/ANPC) or NPR3
Natriuretic peptide receptor (NPR)-A is the primary signaling receptor for atrial natriuretic peptide and brain natriuretic peptide. Ligand binding to NPR-A rapidly activates its guanylyl cyclase domain, but its rate of cGMP synthesis declines with time.
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), found in the atria and ventricles of the heart, respectively, are cardiac hormones that counterbalance the renin-angiotensin-aldosterone system. ANP and BNP bind two distinct cell surface proteins known as the natriuretic peptide clearance receptor and NPR-A/guanylyl cyclase A. The clearance receptor consists of an extracellular domain, a single membrane-spanning region, and only 37 intracellular amino acids.
Receptor-agonist binding causes a reduction in blood volume and, therefore, a reduction in cardiac output and systemic blood pressure. Lipolysis is increased and renal sodium reabsorption is decreased. The overall effect of ANP on the body is to counter increases in blood pressure and volume caused by the renin-angiotensin system.
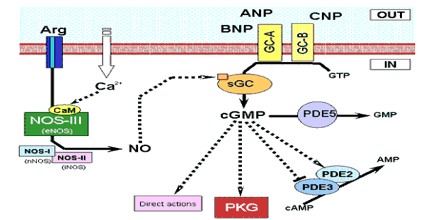
The natriuretic effect of ANP was first reported by De Bold et al. 20 years ago. The effects of ANP and endothelium-derived relaxing factor (EDRF) or nitric oxide (NO) are mediated by cGMP. Cyclic GMP modulates cGMP-gated channels, cGMP-dependent phosphodiesterase, and cGMP-dependent protein kinases (cGK). cGK exists as two major forms, cGK I and cGK II.