Natural aging includes muscle-repairing cells known as myogenic progenitors entering an aged, non-proliferating state known as senescence. As we age, the accumulation of senescent myogenic progenitors severely impairs muscle regeneration. With the accumulation of these senescent cells impeding muscle rebuilding in all aging adults, we must restore the cells’ youthful and functional state in order to preserve healthy muscle.
A team led by the University at Buffalo discovered that a protein named after the mythical land of youth in Irish folklore is effective at reversing aging in skeletal muscle cells. The study, which was published in Science Advances, focuses on the protein NANOG, which is derived from Tr na ng, a place in Irish lore renowned for everlasting youth, beauty, and health.
The physiology of the aging athlete’s muscles is complex. Sarcopenia, or the age-related loss of lean muscle mass, can affect activity level and quality of life. As the population ages, an increasing number of people choose to work much later in life than in the past. This group includes everyone from “weekend warriors” to competitive master athletes. Sports medicine practitioners should be well-versed in both normal physiologic changes and injuries to the aging athlete.
A research team has shown that a protein named for the mythical land of youth in Irish folklore is effective at reversing aging in skeletal muscle cells.
Researchers overexpressed NANOG in myoblasts, which are embryonic precursors to muscle tissue, in a series of experiments. The myoblasts were senescent, which meant they could no longer divide and grow.
Overexpression improved some of the primary characteristics associated with cellular aging, such as autophagy, energy homeostasis, genomic stability, nuclear integrity, and mitochondrial function.
The accumulation of genetic damage during aging is a major driver of cell senescence. Andreadis and colleagues then attempted to evaluate the activation of the DNA damage response, a mechanism by which cells repair DNA. Reduced activation of the DNA damage response would imply that the DNA is in better shape and requires less attention from the cell’s repair mechanisms. NANOG may revert senescent cells to their more youthful, proliferating state by improving the efficiency of DNA repair mechanisms such as the DNA damage response to reduce their activation. Furthermore, their findings suggest that increasing NANOG levels causes more efficient DNA repair with less activation of the DNA damage response.
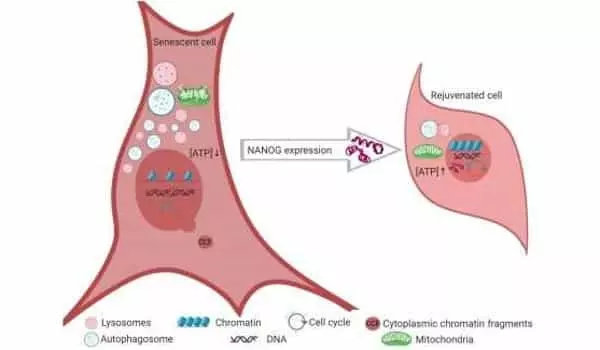
The NANOG protein plays a critical role in the self-renewal of embryonic stem cells, which give rise to all other types of cells in the body. Andreadis and colleagues previously discovered that NANOG restores the ability of mouse muscle progenitor cells to form new muscle even after they have reached a senescent state. These findings prompted a team of researchers from the University of Buffalo to investigate whether these findings apply to human muscle progenitor cells as well as fast-aging mouse skeletal muscle tissue.
Andreadis and colleagues demonstrated in their most recent study that increasing the amount of NANOG present in cells reverses hallmarks of cell senescence in muscle progenitors, restoring their youthful state.
NANOG, most notably, increased the number of muscle stem cells in prematurely aging mice’s muscles. This demonstrated the feasibility of reversing cellular aging in the body without the need to reprogram cells to an embryonic pluripotent state, a process commonly used in stem cell therapy but associated with tumorigenesis.
The Buffalo-based researchers used viruses to increase NANOG levels in human muscle progenitor cells to see how it affected muscle cells. After increasing NANOG levels in senescent cells for 5, 10, or 15 days, the cells re-entered a proliferative state, increasing their numbers. Furthermore, the human muscle progenitor cells had lower levels of an aging cell marker, senescence-associated -galactosidase, indicating that NANOG reverses aging in human muscle progenitor cells.
“Our research focuses on elucidating the mechanisms of NANOG’s actions in the hope of identifying druggable targets in signaling or metabolic networks that mimic NANOG’s anti-aging effects. Finally, the research could lead to new treatments or therapies that can help reverse cellular senescence and help the many people who suffer from age-related disorders “Stelios T. Andreadis, Ph.D., SUNY Distinguished Professor in the Department of Chemical and Biological Engineering at the UB School of Engineering and Applied Sciences, is the study’s corresponding author.