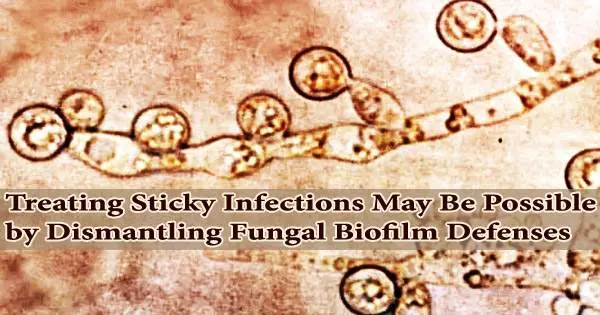
The microbes that harm us frequently have strategies for dodging our attacks. The biofilm matrix, which surrounds hordes of disease-causing organisms, is a sticky, armor-like substance that may be the most effective of these tactics.
This defense is effective, albeit tragically at times. For instance, biofilms are easily formed and invisible on surgical implants and catheters and are extremely resistant to medications that may otherwise be used to treat them. Tens of thousands of lives are lost each year and billions of dollars are spent in the U.S. due to the illnesses they spread.
“There are no approved antimicrobials to treat biofilms. The only way to treat a biofilm is to physically remove it from the body,” says David Andes, a professor of medicine at the University of Wisconsin School of Medicine and Public Health.
Andes and his associates recently identified some of the crucial proteins in Candida albicans biofilms that regulate both how the fungus resists antifungal medications and how it spreads throughout the body in an effort to better understand and attack these formations.
The newly discovered proteins offer prospective therapeutic targets to weaken a pathogen’s antimicrobial defenses, however additional research is required. In fact, the study discovered that Candida that was unable to produce several of these proteins was significantly more vulnerable to the antifungal fluconazole than other Candida.
Andes and his associates recently identified some of the crucial proteins in Candida albicans biofilms that regulate both how the fungus resists antifungal medications and how it spreads throughout the body in an effort to better understand and attack these formations.
There are no approved antimicrobials to treat biofilms. The only way to treat a biofilm is to physically remove it from the body.
Professor David Andes
The newly discovered proteins offer prospective therapeutic targets to weaken a pathogen’s antimicrobial defenses, however additional research is required. In fact, the study discovered that Candida that was unable to produce several of these proteins was significantly more vulnerable to the antifungal fluconazole than other Candida.
A complex soup of substances, including proteins, are secreted by individual cells inside biofilms. Hundreds of these proteins were screened by the researchers using a machine-learning algorithm to find those that were probably involved in the formation and operation of biofilms.
They discovered 63 proteins for additional research. In lab testing, 13 of the mutant Candida strains that the researchers produced were more vulnerable to the antifungal fluconazole.
The proteins in a rat model using venous catheters were also examined by the Andes lab, which specializes in creating animal models to test medication resistance. To assist in the delivery of medications, such as during chemotherapy treatment, these catheters are frequently put into major veins and left there for months.
Catheters are prone to become infection sources because they are within the body for such a long time. Four of the 13 drug-sensitive fungus mutants were evaluated in the rat model, and all four were still susceptible to fluconazole, as had been shown in earlier lab testing.
Normal Candida biofilms are hardly impacted by the antifungal, while mutant fungal populations were reduced by 30 times or more. Not only do biofilms contribute to medication resistance. They have an impact on a pathogen’s complete lifespan.
“The last step in the lifecycle of a biofilm is dispersion. Cells leave the biofilm and spread to other parts of the body,” says Andes. This body-wide dispersal greatly increases the risk from infections.
17 mutants were discovered by the researchers that had an impact on this dispersion process; most of them dispersed more easily. Three of these high-dispersal mutants were tested in rats and caused a more than 10-fold increase in the amount of Candida that reached the kidneys.
Intriguingly, two of the mutations had a mixed clinical outcome: they were both more responsive to antifungal treatment and more likely to spread to the kidney. According to Andes, this combination of partially antibacterial and partially dispersal-controlling functions shows that the proteins play intricate roles in biofilms.
The Andes laboratory has already discovered a medication that can thwart the fungus’s defenses. Recent research has revealed that the antifungal turbinmicin, which Andes and his team discovered in 2020, can inhibit Candida’s capacity to secrete these proteins and other elements of biofilms, making the pathogen more susceptible to medications.