Proteoliposomes, which are also known as artificial lipid bilayer vesicle liposomes, are specialized systems that may contain a wide range of compounds, including chemicals and pharmaceuticals. They are the perfect carriers for delivering compounds within cells thanks to their special qualities.
They must, nevertheless, have the twin properties of high extracellular stability and low intracellular stability. Liposomes that are pH-sensitive are often used, and several methods have been devised to control the stability of liposomes in a condition-dependent manner.
The pH scale, a commonly used indicator of acidity or basicity, ranges from 1 to 14, with 7 representing “neutral,” or like water. A pH below 7 indicates acidity, whereas one above 7 indicates basicity. It’s interesting to note that when exposed to an acidic pH lower than 5.5, pH-sensitive liposomes can be made to leak their contents.
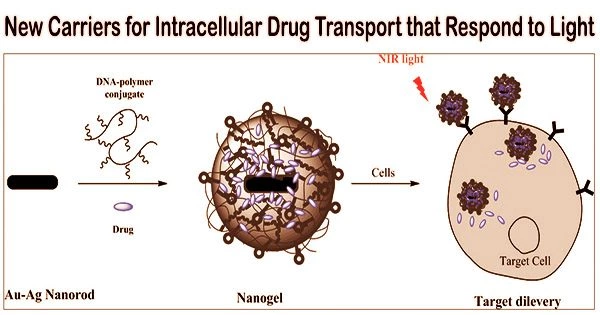
In a new study, a team of researchers, led by Professor Yuki Sudo from Okayama University, Japan, has developed a precise method of releasing contents from pH-sensitive liposomes using light.
“The study presents a novel nanomaterial called light-induced disruptive liposome (LiDL) and applies it to light-controlled intracellular substance delivery,” explains Prof. Sudo. Their work, co-authored by Mr. Taichi Tsuneishi of Okayama University and Prof. Yuma Yamada of Hokkaido University in Japan, was published in the journal Chemical Communications.
The researchers utilized a protein called Rubricoccus marinus Xenorhodopsin (RmXeR), derived from a marine bacterium, to initiate acidification inside liposomes using light. They initially created pH-insensitive proteoliposomes using the lipid hydration method, combining phosphatidylcholine from egg yolk with cholesterol, in order to explore the activity of RmXeR within liposomes without being hampered by pH-dependent features.
The study presents a novel nanomaterial called light-induced disruptive liposome (LiDL) and applies it to light-controlled intracellular substance delivery.
Professor Yuki Sudo
Then, purified RmXeR was incorporated via the dilution method. According to the researchers, when exposed to green light, the pH inside the resulting liposomes decreased from 7.0 to 4.8, making them susceptible to disruption brought on by light.
The researchers next created 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and cholesteryl hemisuccinate-based pH-sensitive proteoliposomes. It’s interesting to note that the researchers tested the liposomes’ ability to release RmXeR before adding it to them by adding calcein, a water-soluble fluorescent dye.
They tracked the fluorescence of calcein and discovered that it fluoresced in a light-dependent manner, demonstrating that the liposomes released chemicals when exposed to light.
These pH-sensitive proteoliposomes, or LiDLs, displayed exceptional stability in the absence of light, maintaining their physiochemical properties when exposed to a temperature of 4°C for two weeks.
The second stage of the investigation involved the introduction of LiDL into mammalian HeLa cells so that the researchers could assess how well they functioned as carriers for delivering chemicals inside the cells. They observed the uptake of LiDLs by cells and discovered that LiDLs, regardless of the presence of RmXeR, increased the uptake of liposomes by HeLa cells. This showed LiDL’s potential as a delivery system for intracellular substances.
The researchers added stearyl octaarginine to LiDL to increase the intracellular delivery effectiveness even further. When compared to LiDL, this improved version, known as LiDL-R8, performed noticeably better.
These results show how useful LiDL is for delivering chemicals and biomolecules into cells. “With LiDL, drug delivery can be controlled by light, potentially leading to advances in various therapies,” emphasizes Prof. Sudo.
The team’s future goals include improving the experimental circumstances and comprehensively characterizing the liposome structure in order to increase the efficiency of content release.
LiDLs actually show a lot of potential as optically adjustable carriers for enhanced medication delivery. These liposomes exhibit outstanding extracellular stability and manageable intracellular instability, providing increased efficacy without producing negative side effects.
“Our study will contribute towards ensuring healthy lives and promoting well-being for people of all ages, which ties in with one of the Sustainable Development Goal (SDG3) of the United Nations,” concludes Prof. Sudo.