Scientists have induced long-term leg growth in adult frogs, who are unable to repair limbs on their own. The frogs regrew a missing leg over months after being exposed to a five-drug cocktail stored in a bioreactor for 24 hours. The replacement legs were sufficiently functioning to allow sensation and mobility.
The chance of restoring function through natural regeneration remains out of reach for millions of individuals who have lost limbs due to causes ranging from diabetes to trauma. Salamanders and superheroes are the only creatures that can regenerate their legs and limbs. Scientists from Tufts University and Harvard University’s Wyss Institute, however, have gotten us one step closer to the aim of regenerative medicine in a study published in the journal Science Advances.
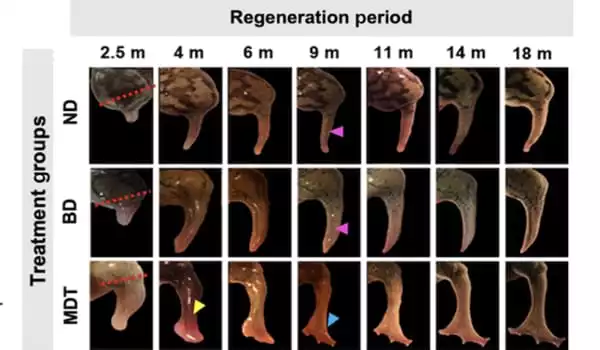
Using a five-drug cocktail administered in a silicone wearable bioreactor dome that seals in the elixir over the stump for only 24 hours, the researchers were able to initiate the recovery of a severed leg in adult frogs, which are naturally unable to regenerate limbs. This quick treatment initiates an 18-month phase of regrowth, restoring a functional limb.
Many species, including salamanders, starfish, crabs, and lizards, are capable of full regeneration of at least some limbs. Flatworms may even be disassembled into parts, with each piece reassembling a whole organism. Humans can repair wounds with new tissue growth, and our livers have an amazing, almost flatworm-like ability to regenerate to full size after a 50% loss.
However, any natural mechanism of regeneration in humans or mammals cannot repair the loss of a large and structurally complex limb, such as an arm or leg. In fact, humans tend to cover significant injuries with an amorphous mass of scar tissue to protect them from future blood loss and infection, as well as to inhibit further growth.
It’s thrilling to see that the medications we chose were assisting in the creation of an almost complete limb. The fact that only a brief exposure to the medications was required to initiate a months-long regeneration process shows that frogs and possibly other animals have dormant regenerative capabilities that can be put into action.
Nirosha Murugan
Kickstarting Regeneration
Tufts researchers induced regeneration in African clawed frogs by enclosing the wound in a silicone cap called a BioDome, which contained a silk protein gel infused with the five-drug cocktail.
Each medicine served a distinct objective, such as reducing inflammation, suppressing collagen development, which would lead to scarring, and supporting the growth of new nerve fibers, blood vessels, and muscle. The combination, as well as the bioreactor, offered a local environment and signals that shifted the balance away from the natural tendency to seal off the stump and toward the regenerative process.
Many of the treated frogs showed remarkable tissue growth, resembling an almost totally functional leg, according to the researchers. The new limbs included a bone structure that was comparable to that of a real limb, a greater complement of interior tissues (including neurons), and many “toes” that sprouted from the end of the limb, albeit without the support of underlying bone.
The regrown limb moved and responded to stimuli such as a touch from a stiff fiber, and the frogs were able to swim through water using it in the same way that a regular frog would.
“It’s thrilling to see that the medications we chose were assisting in the creation of an almost complete limb,” said Nirosha Murugan, a research affiliate at Tufts’ Allen Discovery Center and the paper’s first author. “The fact that only a brief exposure to the medications was required to initiate a months-long regeneration process shows that frogs and possibly other animals have dormant regenerative capabilities that can be put into action.”
The researchers looked at the factors that could lead to long-term growth from a brief intervention. They observed the activation of known molecular pathways that are ordinarily used in a growing embryo to help the body take shape within the first few days following therapy.
Activation of these pathways could allow the limb to carry the burden of tissue growth and organization, similar to how it occurs in an embryo, rather than requiring continual therapeutic intervention over the several months it takes to grow the limb.
How the BioDome Works
Animals that can regenerate naturally are mainly found in aquatic environments. The first stage of growth after a limb loss is the formation of a blastema, a mass of stem cells at the end of the stump that is utilized to gradually restore the missing body part. Within the first 24 hours following an injury, skin cells rapidly cover the wound, safeguarding the reconstructing tissue beneath.
“Mammals and other regenerating animals’ injuries are frequently exposed to air or make touch with the ground, and they can take days to weeks to cover up with scar tissue,” said David Kaplan, Stern Family Professor of Engineering at Tufts and co-author of the study. “Using the BioDome cap in the first 24 hours helps mimic an amniotic-like environment which, along with the right drugs, allows the rebuilding process to proceed without the interference of scar tissue.”
Next Steps in Frogs and Mammals
Previous research by the Tufts team with the BioDome shown a large degree of limb development caused by a single medication, progesterone. The resulting limb, however, grew like a spike and was very different from the more regularly formed, functioning limb achieved in the current study.
The five-drug cocktail represents an important step toward the restoration of fully functional frog limbs, implying that further research into drug and growth factor combinations could lead to regrown limbs that are even more functionally complete, with normal digits, webbing, and more detailed skeletal and muscular features.
“We’ll be evaluating how this treatment could apply to mammals next,” said corresponding author Michael Levin, Vannevar Bush Professor of Biology in the School of Arts and Sciences, director of Tufts’ Allen Discovery Center, and Wyss Institute affiliate faculty member.
“Covering the open wound with a liquid environment beneath the BioDome, together with the correct pharmacological cocktail, could provide the crucial first signals to begin the regenerative process in action,” he explained. “It’s a method based on reactivating dormant, innate anatomical patterning systems rather than micromanaging complex growth because mature animals still have the information needed to build their bodies.”