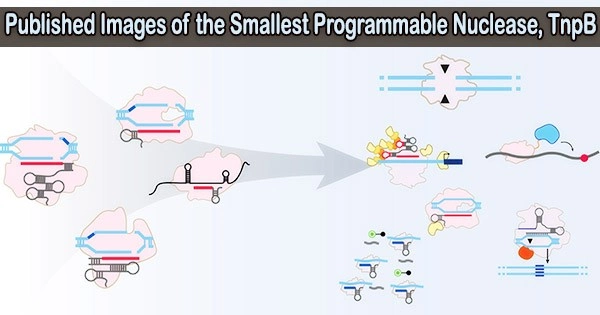
Cryo-electron microscopy was used by a team led by Professor Virginijus Šikšnys from Vilnius University Life Sciences Center (VU LSC) in collaboration with Professor Guillermo Montoya’s group at the Novo Nordisk Foundation Center for Protein Research (CPR) at the University of Copenhagen to determine the structure of TnpB.
The article “TnpB structure reveals the minimal functional core of Cas12 nuclease family” was published in Nature.
Gene scissors or CRISPR-Cas nucleases, such as Cas9 or Cas12, have completely changed the way that genome editing is done. They make it possible to precisely alter genomes and fix mutations that cause disease. The size of Cas9 or Cas12, which is already utilized in gene therapy, restricts their ability to be delivered to target cells by Adeno-associated viruses (AAV).
Researchers from VU LSC previously announced the discovery of a novel class of programmable nucleases dubbed TnpBs, which are linked to transposons, mobile genomic elements. They proved that TnpB is the smallest programmable nuclease that can be used for effective gene editing, but they didn’t know how it was structurally organized or worked.
First of all the relevant research object and the collaboration of VU LSC biochemists, molecular biologists, bioinformaticians, and colleagues from NNF-CPR at the University of Copenhagen. But most importantly, we were able to conduct this research in Lithuania using the cryo-electron microscope available at VU LSC.
Professor Virginijus Šikšnys
“The new Nature paper that just came out is a result of a consistent and long-term effort that demonstrates the potential of Lithuanian scientists in the field of life sciences and their ability to be among the leaders in this field. This research has revealed the structure and mechanism of TnpB gene scissors, which creates a basis for further targeted engineering of the TnpB complex to transform it into a therapeutic tool for treating genetic disease,” says Professor V. Šikšnys.
The smallest programmable endonuclease, TnpB, had its ternary structure determined in the current study using cryo-electron microscopy (cryo-EM), which, together with biochemical experiments, clarified how TnpB gene scissors can precisely recognize and cut DNA targets.
The TnpB protein and a lengthy RNA molecule work together to create a complex three-dimensional structure that not only aids in identifying the DNA target but also regulates the DNA-cutting activity of TnpB.
TnpB is the forerunner of the Cas12 nuclease family and is the structural-functional core of the Cas12, according to a comparison of structure and bioinformatic research.
As noted by one of the article’s authors, Dr. Giedrius Sasnauskas, the success of this research was determined by several factors.
“First of all the relevant research object and the collaboration of VU LSC biochemists, molecular biologists, bioinformaticians, and colleagues from NNF-CPR at the University of Copenhagen. But most importantly, we were able to conduct this research in Lithuania using the cryo-electron microscope available at VU LSC,” says the researcher.