Zirconia-based catalysts are a type of heterogeneous catalyst that contains zirconium oxide (ZrO2) as the active component. Zirconia is a stable and inert material that has been widely used as a support for various catalysts due to its high surface area, thermal stability, and resistance to corrosion.
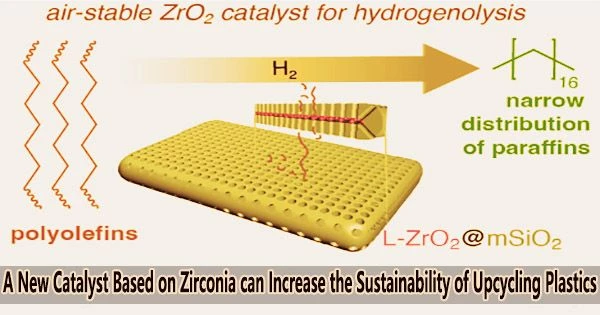
A new type of catalyst breaks down polyolefin plastics into new, useful products. The goal of this project is to recover value that is lost when plastics are thrown away while also reducing the amount of plastic trash and its negative effects on the environment.
A team from the Institute developed the catalyst for Cooperative Upcycling of Plastic (iCOUP), a U.S. Department of Energy, Energy Frontier Research Center. The effort was led by Aaron Sadow, the director of iCOUP, scientist at Ames National Laboratory, and professor at Iowa State University; Andreas Heyden, professor at the University of South Carolina; and Wenyu Huang, scientist at Ames Lab and professor at Iowa State. They demonstrated that the novel catalyst, which is only formed of materials found on Earth, can dissolve aliphatic hydrocarbons’ carbon-carbon (CC) bonds.
Organic substances known as aliphatic hydrocarbons exclusively include carbon and hydrogen. Aliphatic hydrocarbons, or polyolefins, are strong materials made of long chains of carbon atoms bonded together. These materials are a big part of the plastic waste crisis.
Being able to take an air-exposed metal oxide, heat it with an alkane, and generate an organometallic is a really powerful reaction that enables this kind of hydrogenolysis process. It potentially could enable lots of interesting catalytic transformations of hydrocarbons that were previously not considered.
Aaron Sadow
Wenyu Huang said, “More than half of produced plastics so far are polyolefin based.”
In the current world, polyolefin plastics are utilized in a wide variety of products, including electronics, shrink wrap, other packaging materials, and containers for liquids like milk or detergents.
Nonetheless, polyolefins are among the most challenging plastics to recycle, and new strategies are required, as Andreas Heyden noted. Upcycling is one such viable recycling substitute. The materials used in this strategy are chemically transformed into products with increased value.
One way to upcycle polyolefins is a chemical process called hydrogenolysis. By severing CC bonds and introducing hydrogen, a catalyst separates molecular chains during this process. Aaron Sadow claims that platinum-based catalysts, in particular, are frequently utilized in hydrogenolysis. Due to its efficacy, platinum is utilized in many different catalytic reactions despite the fact that it is expensive due to its low quantity in the earth’s crust.
To address both challenges of sustainability and economy, Heyden said, “We thought we’d be able to use earth-abundant elements to create much cheaper catalytic materials, and by assembling these elements in a certain way we might achieve a high selectivity and still very good activity.”
Zirconia-based catalysts have been used in various industrial applications, such as in the production of chemicals, petrochemicals, and environmental remediation. They can catalyze a wide range of reactions, including acid-catalyzed reactions, dehydration reactions, oxidation reactions, and hydrogenation reactions.
The team discovered that zirconia, an earth-abundant metal oxide, can cut CC bonds in aliphatic hydrocarbon polymers at about the same speed of precious metal catalysts.
“We were surprised that we could do hydrogenolysis of CC bonds, using zirconium oxide as the catalyst. The conventional paradigm is that zirconia is not very reactive on its own,” said Sadow.
The catalyst’s structure, which was created by Wenyu Huang and his team, is the secret to its success.
“In this architecture, ultrasmall zirconia nanoparticles are embedded between two plates of mesoporous silica. The two silica plates are fused, with the zirconia embedded in the middle, like a sandwich,” Huang said. “The pores in the silica provide access to the zirconia, while the sandwich-like structure protects the zirconia nanoparticles from sintering or crystallization, which would make them less effective.”
The task of simulating the reaction and figuring out where and how the active site functions in reaction conditions fell to Heyden’s team.
“And so for that we do both quantum chemical modeling of the catalyst and the chemical reactions together with some classical chemical reactor modeling,” he explained. “And here we really saw the importance of that amorphous zirconia structure.”
Sadow claims that the inspiration for researching zirconia in hydrogenolysis came from groundbreaking work on polymer depolymerization done in the late 1990s utilizing zirconium hydrides.
“Harnessing zirconium hydrides for hydrogenolysis is really nice chemistry,” he said. “The problem is those zirconium organometallic species are really air and water sensitive. So they have to be handled under the cleanest of conditions. Typically polymer waste is not pure and isn’t supplied as a clean and perfectly dry starting material. Using a zirconium hydride catalyst, you’d have to really worry about impurities that inhibit the chemistry.”
The newly created zirconia material is merely heated in vacuum prior to the reactions, and it remains active throughout the hydrogenolysis procedure.
“Zirconium oxide is easily handled in air and then activated. It doesn’t require any kind of really specialized conditions, which was also exciting,” Sadow said.
“Being able to take an air-exposed metal oxide, heat it with an alkane, and generate an organometallic is a really powerful reaction that enables this kind of hydrogenolysis process. It potentially could enable lots of interesting catalytic transformations of hydrocarbons that were previously not considered.”