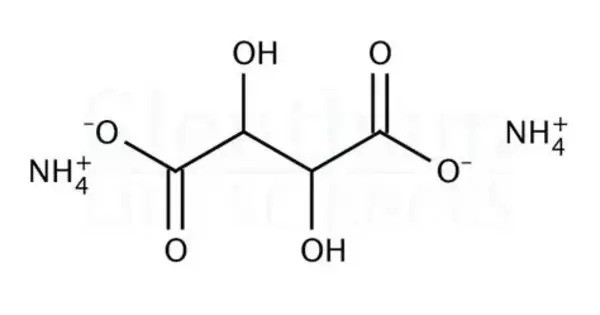
Ammonium tartrate is a chemical compound with the chemical formula (NH4)2C4H4O6. It is a white crystalline salt composed of ammonium and tartrate ions. This is an organic ammonium salt of tartaric acid. It is commonly used in analytical chemistry, especially in Fehling’s solution as a complexing agent for metal ions. This compound is soluble in water and has moderate stability under standard conditions. It can act as a buffering agent and is sometimes used in fertilizers or as a food additive.
Ammonium tartrate occurs in two forms: the normal salt and the acid salt (ammonium hydrogen tartrate). It is non-toxic in small quantities but should be handled carefully to avoid irritation. It is biodegradable and relatively environmentally safe.
Synthesis
Ammonium tartrate can be prepared by the reaction of tartaric acid and ammonium carbonate.
Properties
Ammonium tartrate forms colorless crystals that slowly release ammonia if exposed to air. Easily soluble in water, also soluble in alcohol.
- Chemical formula: C4H12N2O6
- Molar mass: 184.148 g·mol−1
- Appearance: colorless crystals
- Density: 1.601 g/cm3
- Boiling point: 399.3 °C
- Solubility in water: soluble
Occurrence and Sources
Ammonium tartrate does not occur naturally in significant quantities. It is a synthetic compound formed by the neutralization of tartaric acid with ammonia or ammonium hydroxide. However, related tartrate compounds occur in nature:
Tartaric acid is found naturally in grapes, bananas, and tamarinds. Natural tartrates, like potassium hydrogen tartrate (cream of tartar), are by-products in winemaking.
Uses
The compound is used in textile industry and in medicine. Also can be used as an analytical reagent and an intermediate in organic synthesis.
- Analytical chemistry: As a reagent or buffer.
- Electroplating: As a complexing agent in some metal plating baths.
- Fertilizer: Occasionally used as a nitrogen source.
- Catalysis: In some chemical syntheses.
- Pharmaceuticals & food: Rarely used, but related tartrates are common in food as acidity regulators.