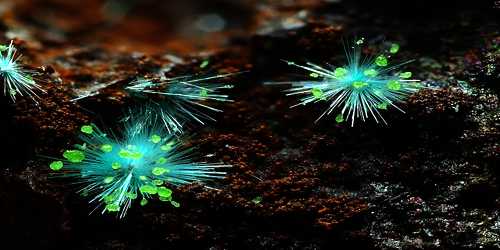
Agardite is a mineral group consisting of agardite-(Y), agardite-(Ce), agardite-(Nd) and agardite- They comprise a group of minerals that are hydrous hydrated arsenates of rare-earth elements (REE) and copper, with the general chemical formula (REE,Ca)Cu6(AsO4)3(OH)6·3H2O.
Agardite-(La) was named by Peter J. Modreski in 1983 as an analogy to agardite-(Y), with its rare-earth content dominated by lanthanum. This mineral belongs to the mixite group. Agardite-(Y) was named after Jules Agard, a geologist at the Bureau de Recherches Geologiques et Minieres in Orleans, France. This mineral belongs to the mixite group.
Yttrium, cerium, neodymium, lanthanum, as well as the trace to minor amounts of other REEs, are present in their structure.

Global Distribution
- Agardite-(La) is distributed in the following places: USA, Greece, England, Italy, and Germany.
- Agardite-(Y) is distributed in the following places: Spain, Germany, Italy, France, UK, Wales, Japan, Australia, USA, Mexico, Morocco, and Congo.
General Information:
- Category: Arsenate minerals
- Formula: (REE,Ca)Cu6(AsO4)3(OH)63H2O.
- Crystal system: Hexagonal
Identification
- Color: Yellow-green
- Crystal habit: Acicular
- Cleavage: None
- Fracture: Conchoidal
- Mohs scale hardness: 3–4
- Luster: Vitreous
- Streak: Greenish white
- Specific gravity: 3.7 (measured), 3.775 (calculated)
Occurrence of Agardite-(Y)
Agardite-(Y) occurs in the oxidation zone of some copper deposits. It is often associated with minerals such as azurite, chrysocolla, malachite, cuprite, copper, quartz, and mimetite.
Occurrence of Agardite-(La)
Agardite-(La) occurs in small amounts in the oxidized zone of hydrothermal mineralized breccia and polymetallic mineral deposits.
It is often associated with minerals such as fluorite, bastnaesite, barite, quartz, chrysocolla, azurite, malachite, mimetite, vanadinite, conichalcite, mottramite, cerussite, smithsonite, wulfenite, aurichalcite, hydrozincite, azurite, cuprian adamite, chrysocolla, zincaluminite, gibbsite, and calcite.
Information Source: