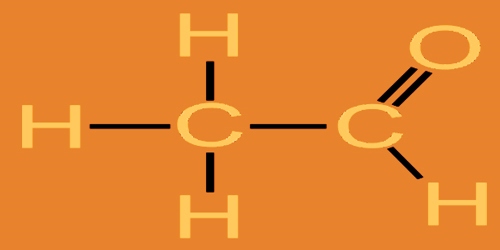
Acetaldehyde (CH3CHO), also known as ‘ethanal’, is a widespread, naturally occurring, colorless and flammable liquid with a suffocating smell. It is sometimes abbreviated by chemists as MeCHO (Me = methyl), and it is one among the foremost important aldehydes, occurring widely in nature and being produced on a large scale in the industry. It is used in the synthesis of 1-butanol (n-butyl alcohol), ethyl acetate, perfumes, flavorings, aniline dyes, plastics, synthetic rubber, and other chemical compounds as the starting material.
Acetaldehyde is found in various plants, ripe fruits, vegetables, cigarette smoke, gasoline, and diesel exhaust. It also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and may be a contributing reason behind hangover after alcohol consumption. Pure acetaldehyde is a colorless, flammable liquid with a pungent, fruity odor; it boils at 20.8 °C (69.4 °F).
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. This chemical compound is commonly used as a flavoring agent and as an intermediate in alcohol metabolism in the manufacture of acetic acid, perfumes, dyes, and medicines. Acute exposure to their vapors leads to hair, skin, and respiratory tract irritation. Acetaldehyde is “one of the most frequently found air toxins with cancer risk greater than one in a million”.
The Swedish pharmacist/chemist Carl Wilhelm Scheele (1774) first encountered acetaldehyde. The dominant method for acetaldehyde production today is the Wacker method, developed between 1957 and 1959, which catalyzes the oxidation of ethylene to acetaldehyde. The main method of production is the oxidation of ethylene by the Wacker process, which involves oxidation of ethylene using a homogeneous palladium/copper system:
2 CH2=CH2 + O2 → 2 CH3CHO
The Wacker-Hoechst direct oxidation process’ world potential reached 2 million tons per annum in the 1970s.
Acetaldehyde is formed by the alcohol dehydrogenase action on ethanol and is a little more toxic than ethanol. Traditionally, acetaldehyde was produced by the partial dehydrogenation of ethanol:
CH3CH2OH → CH3CHO + H2
Ethanol vapor in this endothermic process is passed over a copper-based catalyst at 260–290 ° C. Due to the value of the hydrogen co-product, the process was once attractive but it is not economically viable in modern times.
Acetaldehyde is miscible altogether proportions with water and most typical organic solvents, e.g., acetone, benzene, ethyl alcohol, inhalation general anesthetic, gasoline, paraldehyde, toluene, xylenes, turpentine, and ethanoic acid. Acetaldehyde is a vital precursor to pyridine derivatives, pentaerythritol, and crotonaldehyde. No serious cases of acute intoxication are recorded; Acetaldehyde naturally breaks down within the frame. Avoid exposure to air any longer than necessary so as to prevent peroxide formation; stable under recommended storage conditions. Acetaldehyde is an irritant of the skin, eyes, mucous membranes, throat, and respiratory tract. In addition, it is transferred to the fetal side when acetaldehyde is present in the maternal perfusate, reaching approximately 50 percent of the maternal level.
Information sources: