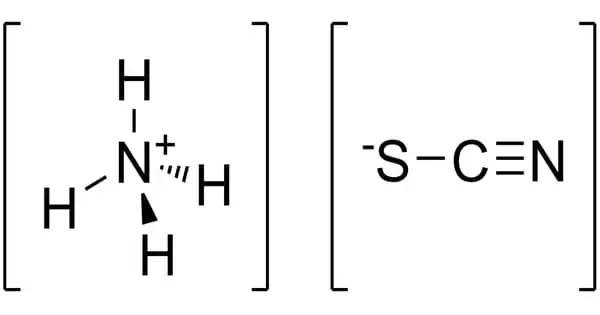Ammonium thiocyanate has the formula NH4SCN and is an inorganic chemical. It is the ammonium cation and thiocyanate anion salt. It is a colorless crystalline substance that is very poisonous and soluble in water. The odorless inorganic substance has a wide range of industrial and consumer applications, including chemical analysis, photography, and fertilizer.
The crystalline solid ammonium thiocyanate is colorless. It dissolves in water. The primary risk is the threat to the environment. Immediate action should be done to limit its environmental spread. It is used in chemical analysis, photography, as a fertilizer, and a variety of other applications.
Properties
Ammonium thiocyanate is a toxic, colorless, crystalline solid inorganic compound with the molecular formula CHNS.H3N or CH4N2S. It is the salt of the ammonium cation and the thiocyanate anion. It is produced as dry powder, hygroscopic crystals, liquid, pellets, and large crystals.
- Chemical formula: NH4SCN
- Molar mass: 76.122 g/mol
- Appearance: Colorless hygroscopic crystalline solid
- Density: 1.305 g/cm3
- Melting point: 149.5 °C (301.1 °F; 422.6 K)
- Boiling point: 170 °C (338 °F; 443 K) (decomposes)
- Solubility in water: 128 g/100 mL (0 °C)
- Solubility: soluble in liquid ammonia, alcohol, acetone

Preparation
Carbon disulfide reacts with water ammonia to produce ammonium thiocyanate. As an intermediate in this process, ammonium dithiocarbamate is produced, which upon heating decomposes to ammonium thiocyanate and hydrogen sulfide:
CS2 + 2 NH3(aq) → NH2C(=S)SNH4 → NH4SCN + H2S
Ammonium thiocyanate is very soluble in water and ethanol; soluble in acetone; highly soluble in liquid ammonia and liquid sulfur dioxide; and mildly soluble in acetonitrile.
Production method
Carbon disulfide method: mix carbon disulfide and a little amount of ammonia with water for around 20 hours at a pressure of 5.88 105Pa and a temperature of 100 °C to produce ammonium thiocyanate. To remove the hydrogen sulfide, the reaction solution was evaporated under decreased pressure. At 105 °C, use ammonium sulfide to remove iron and heavy metals, then filter and concentrate the filtrate under reduced pressure for cooling crystallization in the mold, and then isolate using centrifugation, dying to yield ammonium thiocyanate.
CS2 + 3NH3 → NH4SCN + NH4HS
NH4HS → NH3 ↑ + H2S ↑
Sulfur method: add the appropriate amount of water and sulfur powder to the reactor and mix to form a paste; add gently graded solid sodium cyanide for reaction to produce sulfur sodium cyanide at a temperature of around 110 °C. The reaction is then aided by the addition of solid ammonium chloride to produce sulfur ammonium cyanate. The reaction mixture was then added to barium thiocyanate; the impurities were removed for clarification; the supernatant was evaporated under reduced pressure with concentration to precipitate out the sodium chloride; and finally, the ammonium thiocyanate was filtered, cooled and crystallized, separated, and dried.
NaCN + S → NaSCN
NaSCN + NH4Cl → NaCl + NH4SCN
Uses
Ammonium thiocyanate is used in the production of herbicides, thiourea, and transparent artificial resins; matches; as a stabilizing agent in photography; in various rustproofing compositions; as an adjuvant in textile dyeing and printing; as an oil field tracer; in the separation of hafnium from zirconium; and in titrimetric analyses.
Colorimetry can also be used to evaluate the iron content of soft drinks using ammonium thiocyanate.
Following the isolation of quinine from the neutral, aqueous, sulphate solution, ammonium thiocyanate can be employed to extract quinidine from liquors. The salt is added to the heated solution, and the resulting sticky solid is separated from the liquid. The solid is then refluxed with methanol, which dissolves most of the impurities, leaving the quinidine thiocyanate as a crystalline solid of 90 – 95% purity.