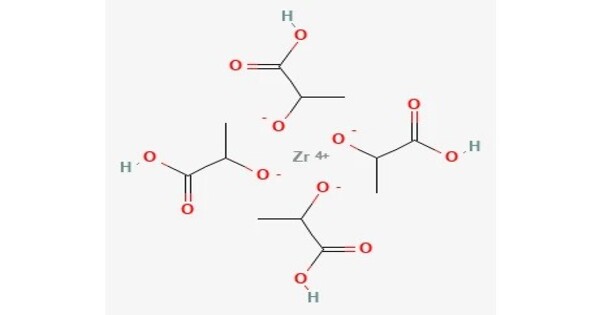
Zirconium lactate is the zirconium salt of lactic acid. It is used in some deodorants. It is a chemical compound formed by the reaction of zirconium salts with lactic acid. Zirconium carboxylates adopt highly complex structures and are heterogeneous compositions with the approximate formula Zr(OH)4-n(O2CCHOHCH3)n(H2O)x where 1 < n < 3. It is often used in antiperspirants and other cosmetic products.
Zirconium lactate is commonly used in antiperspirants due to its ability to form complexes that block sweat glands. It is used in various formulations to enhance the product’s stability and efficacy. It can cause irritation to the skin and eyes. Proper protective equipment should be used when handling.
Properties
It is a colourless solid. Typically, it appears as a white to off-white powder or crystalline solid. The specific melting point can vary depending on the degree of hydration and purity.
- Molecular Formula: C6H10O6Zr
- Molecular Weight: Approx. 285.37 g/mol (for the anhydrous form)
- Solubility: Soluble in water and organic solvents such as alcohol.
Safety
Its LD50 >10 g/kg). It is suspected of causing zirconium granulomas (a form of skin irritation) in a small number of users.
Chemical Reactions
Complex Formation: Zirconium lactate can form complexes with other organic and inorganic substances, which can be utilized in various applications. It should be stored in a cool, dry place away from incompatible substances.
Uses
It is also used in the petroleum industry as a cross-linking agent to prepare gels for fracturing fluids, fluids which are pumped into an oil-bearing rock formation to cause cracks in the rock and so to allow the oil to be extracted. It may be prepared by treating zirconium oxide with lactic acid.