Around 3 billion years ago, an enzyme appeared that would drastically alter the chemical composition of our planet and set in motion an unprecedented surge in biological activity. This enzyme harnessed solar energy to power the thermodynamically and chemically demanding a water-splitting reaction. In doing so, it provided biology with an infinite supply of reducing equivalents required to convert carbon dioxide into organic molecules of life while also producing oxygen to convert our planetary atmosphere from anaerobic to aerobic.
Progress has been made toward the production of hydrogen from sunlight. A new enzyme-like molecular catalyst for water oxidation has been developed by chemists. Humanity is confronted with a critical challenge: managing the transition to a carbon-neutral energy economy.
Hydrogen is regarded as a promising replacement for fossil fuels. It can be made from water and electricity. Green hydrogen is created when electricity is generated from renewable sources. However, it would be even more sustainable if hydrogen could be produced directly from sunlight’s energy.
In nature, light-driven water splitting takes place during photosynthesis in plants. Plants use a complex molecular apparatus for this, the so-called photosystem II. Mimicking its active centre is a promising strategy for realising the sustainable production of hydrogen. A team led by Professor Frank Würthner at the Institute of Organic Chemistry and the Center for Nanosystems Chemistry at Julius-Maximilians-Universität Würzburg (JMU) is working on this.
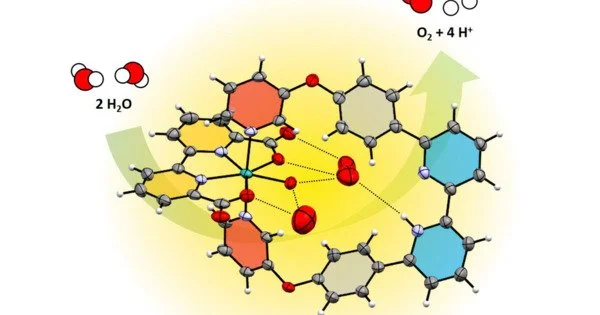
This success was made possible because our doctoral student Niklas Noll created an artificial pocket around the Ruthenium catalyst. Therein, the water molecules for the desired proton-coupled electron transfer are arranged in front of the ruthenium centre in a precisely defined arrangement, similar to what happens in enzymes.
Frank Würthner
Water splitting is not trivial
Water (H2O) consists of one oxygen and two hydrogen atoms. The first step of water splitting is a challenge: to release the hydrogen, the oxygen must be removed from two water molecules. To do this, it is first necessary to remove four electrons and four protons from the two water molecules.
This oxidative reaction is not trivial. Plants use a complex structure to catalyze this process, consisting of a cluster with four manganese atoms over which the electrons can spread. Würthner’s team has developed a similar solution within their first breakthrough published in the journals Nature Chemistry and Energy & Environmental Science in 2016 and 2017, a kind of “artificial enzyme” that can manage the first step of water splitting. This water oxidation catalyst, which consists of three Ruthenium centres interacting in a macrocyclic architecture, successfully catalyzes the thermodynamically-demanding process of water oxidation.
Success with an artificial pocket
Now, chemists at JMU have succeeded in making the sophisticated reaction take place efficiently on a single ruthenium centre. In the process, they have even achieved similarly high catalytic activities as in the natural model, the photosynthetic apparatus of plants.
“This success was made possible because our doctoral student Niklas Noll created an artificial pocket around the Ruthenium catalyst. Therein, the water molecules for the desired proton-coupled electron transfer are arranged in front of the ruthenium centre in a precisely defined arrangement, similar to what happens in enzymes,” says Frank Würthner.
The long-term goal of the Würzburg group is to integrate the water oxidation catalyst into an artificial device that splits water into oxygen and hydrogen with the help of sunlight. This will take some time, as the catalyst must be coupled with other components to form a functioning overall system – with light-harvesting dyes and with so-called reduction catalysts.