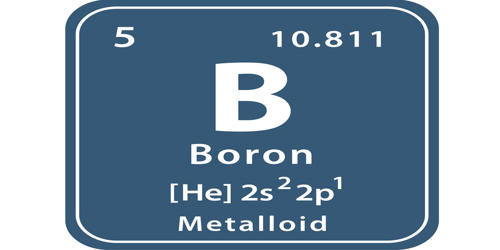Valence Electron
Definition
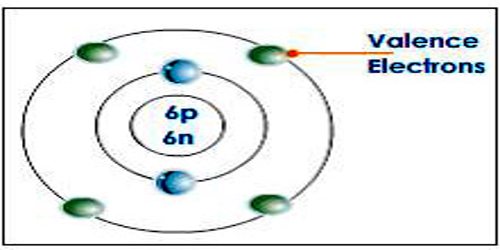
Valence electron is an electron in one of the outer shells of an atom that can participate in forming chemical bonds with other atoms. The presence of valence electrons can determine the element’s chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. For a main group element, a valence electron can exist only in the outermost electron shell; in a transition metal, a valence electron can also be in an inner shell.
A valence electron is an electron that is the most likely to be involved in a chemical reaction. They are typically the electrons with the highest value of the principle quantum number, n. The simplest way to identify the valence electrons is to look for the highest number in the electron configuration of an atom (the principle quantum number).
Similar to an electron in an inner shell, a valence electron has the ability to absorb or release energy in the form of a photon. An energy gain can trigger an electron to move (jump) to an outer shell; this is known as atomic excitation. Or the electron can even break free from its associated atom’s valence shell; this is ionization to form a positive ion. When an electron loses energy, then it can move to an inner shell which is not fully occupied.
Examples: Magnesium’s ground state electron configuration is 1s22s2p63s2, the valence electrons would be the 3s electrons because 3 is the highest principle quantum number.
Electron Configuration
An electron configuration is the arrangement of electrons around the nucleus of an atom. Each atom has its own position on the periodic table. The atomic number of an atom in the ground state is the same as the number of electrons. For example, sodium has an atomic number of 11 and magnesium has an atomic number of 12. So, sodium should contain 11 electrons in its electron configuration and magnesium should contain 12.
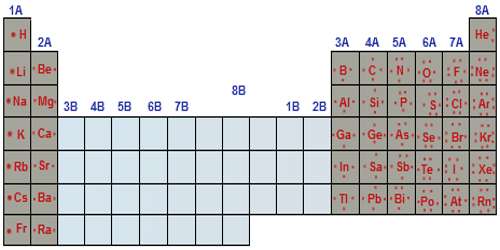
Each atom occupies an orbital in a certain order. This is called the spdf notation. The periodic table is divided into s, p, d, and f blocks.
However, transition elements have partially filled (n − 1)d energy levels, that are very close in energy to the ns level. So as opposed to main group elements, a valence electron for a transition metal is defined as an electron that resides outside a noble-gas core. Thus, generally, the d electrons in transition metals behave as valence electrons although they are not in the valence shell. For example, manganese (Mn) has configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d5; this is abbreviated to [Ar] 4s2 3d5, where [Ar] denotes a core configuration identical to that of the noble gas argon. In this atom, a 3d electron has energy similar to that of a 4s electron, and much higher than that of a 3s or 3p electron. In effect, there are possibly seven valence electrons (4s2 3d5) outside the argon-like core; this is consistent with the chemical fact that manganese can have an oxidation state as high as +7 (in the permanganate ion: MnO−4).
Chemical Reactions of Valence Electron
The electrons in the outermost shell are the valence electrons–the electrons on an atom that can be gained or lost in a chemical reaction. Since filled d or f subshells are seldom disturbed in a chemical reaction, we can define valence electrons as follows: The electrons on an atom that are not present in the previous rare gas, ignoring filled d or f subshells.
Gallium has the following electron configuration.
Ga: [Ar] 4s2 3d10 4p1
The 4s and 4p electrons can be lost in a chemical reaction, but not the electrons in the filled 3d subshell. Gallium therefore has three valence electrons.
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. The number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
The most reactive kind of metallic element is an alkali metal of group 1 (e.g., sodium or potassium); this is because such an atom has only a single valence electron; during the formation of an ionic bond which provides the necessary ionization energy, this one valence electron is easily lost to form a positive ion (cation) with a closed shell (e.g., Na+ or K+). An alkaline earth metal of Group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive ion with a closed shell (e.g., Mg2+).
Valence electrons are also responsible for the electrical conductivity of an element; as a result, an element may be classified as a metal, a nonmetal, or a semiconductor (or metalloid).
Reference: