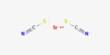
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. is a chemical compound primarily known for its role in the production of Portland cement. It is one of the main phases formed during the manufacturing of cement clinker and significantly influences the setting behavior of cement. It does not occur in nature, but is an important mineral phase in Portland cement.
Properties
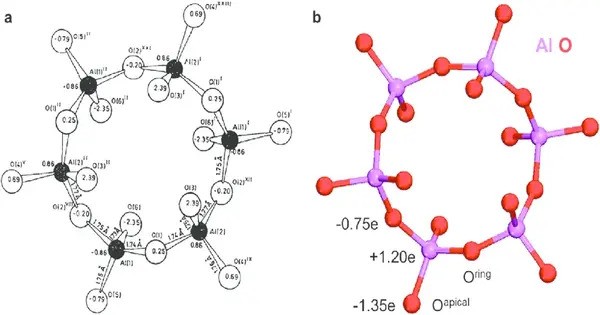
Tricalcium aluminate forms upon heating a 3:1 mixture of calcium oxide and aluminium oxide above 1300 °C. The crystals are cubic, with unit cell dimension 1.5263 nm and has density 3064 kg·m−3. It melts with decomposition at 1542 °C. The unit cell contains 8 cyclic Al6O1818− anions, which can be considered to consist of 6 corner sharing AlO4 tetrahedra.
- Chemical formula: Ca3Al2O6, or 3CaO·Al2O3
- Molar mass: 270.193 g/mol
- Density: 3.064 g/cm3
- Melting point: 1,542 °C (2,808 °F; 1,815 K)
Role in Cement and Hydration
- Hydration Behavior: Tricalcium aluminate reacts very rapidly with water, forming calcium aluminate hydrates. This reaction is exothermic and can cause flash setting (very fast stiffening) unless gypsum is added to regulate the reaction.
- Interaction with Gypsum: Gypsum (CaSO₄·2H₂O) slows down the hydration of C₃A by forming ettringite (a calcium sulfoaluminate hydrate), allowing the cement to remain workable for longer.
- Effect on Concrete Durability: C₃A contributes to sulfate attack vulnerability; cement with lower C₃A content is more resistant to sulfate environments.
Occurrences
- Natural Occurrence: Rare in nature; tricalcium aluminate is not commonly found as a natural mineral.
- Industrial Production: Found in Portland cement clinker, typically in quantities of 5–15% by mass. Formed during the high-temperature reaction of limestone (CaCO₃) and alumina-rich materials (such as clay or bauxite) in the cement kiln.
Applications
- Tricalcium aluminate is essential in determining the early setting and heat evolution characteristics of cement.
- Important for controlling the workability and durability of concrete through proper formulation and use of admixtures.