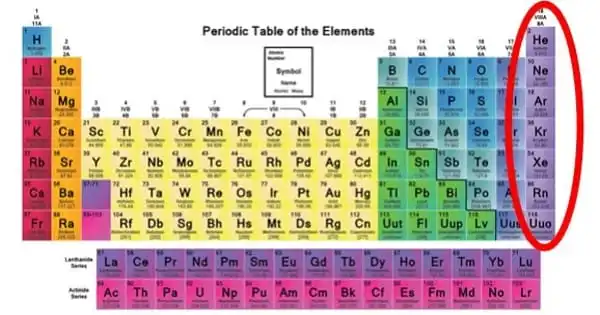
Under a particular set of conditions, an inert gas is a gas that does not conduct chemical reactions. It is a gas that does not react chemically with other chemicals and so does not form chemical compounds. Because noble gases frequently do not react with many things, they were previously referred to as inert gases. The term “inert” refers to being chemically inactive. These gases are also referred to as “noble gases.” Group 18 is frequently referred to as the zero group. As a result, elements of group 18 are often known as zero group elements.
In general, inert gases are employed to prevent undesirable chemical reactions from damaging a sample. These unfavorable chemical reactions include frequently oxidation and hydrolysis reactions with oxygen and moisture in the air. Because numerous noble gases can be made to react under certain conditions, the term “inert gas” is context-dependent.
Because of their natural abundance (78.3 percent N2, 1 percent Ar in the air) and low relative cost, purified argon and nitrogen gases are the most commonly used inert gases.
In contrast to noble gases, inert gases are not always elemental and are frequently composite gases. The tendency for non-reactivity, like that of noble gases, is owing to the valence, or outermost electron shell, being complete in all inert gases. Because noble gases and other “inert” gases can react to form compounds, this is a trend rather than a law.
Properties
They are normally colorless, odorless, and tasteless gases that are non-flammable. For many decades, they have been placed in the standard periodic table’s zero groups because they are assumed to be fully non-bonding to other atoms, such that the atoms of the noble gases do not react with the atoms of any other elements to form a new chemical compound.
Production
With the exception of helium, which is isolated from a few natural gas sources rich in this element via cryogenic distillation or membrane separation, the inert gases are derived through fractional distillation of air. Purified inert gas must be created on-site by specialized generators for specialized purposes. Chemical tankers and product haulers frequently employ them (smaller vessels). For laboratories, there are additional benchtop specialist generators available.
Applications
Because of their non-reactive qualities, inert gases are frequently used to prevent unwanted chemical reactions. To remove oxygen gas, food is enclosed in inert gas. This inhibits the growth of microorganisms. In normal air, it also resists chemical oxidation by oxygen. Edible oil rancidification (induced by oxidation) is one example. In contrast to active preservatives such as sodium benzoate (an antibacterial) or BHT, inert gases are utilized as a passive preservative in food packaging (an antioxidant).
To avoid decay, historical documents can also be kept in an inert atmosphere. The original documents of the United States Constitution, for example, are kept in humidified argon. Previously, helium was utilized, but it was less suited since it diffuses out of the case faster than argon.
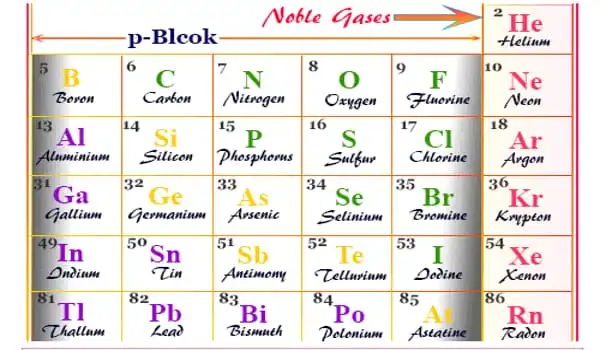
In the chemical industry, inert gases are frequently employed. In a chemical manufacturing plant, reactions can be carried out under inert gas to reduce the risk of fire or undesired reactions. Transfer lines and vessels in such factories and oil refineries can be purged with inert gas to prevent fires and explosions. Chemists conduct investigations on air-sensitive substances at the bench scale using air-free methodologies devised to handle them under inert gas. Helium, neon, argon, krypton, xenon, and radon are inert gases.
Toxicity and Combustibility
Inert gases are harmless and non-combustible due to their extremely low reactivity. As a result, they are advantageous in many situations where other gases would be hazardous. They can also be used to prevent or inhibit potentially harmful effects. Inert gas fire suppression systems, for example, work by displacing the oxygen required to sustain a fire, which can limit the spread of a fire or even extinguish it entirely. In settings where electrical fires constitute a substantial risk, the use of inert gas fire suppression devices is particularly frequent.