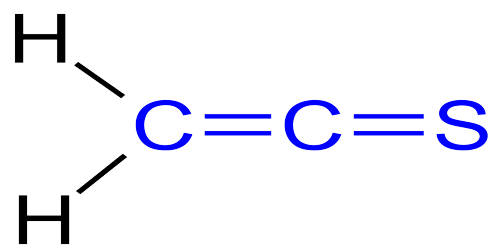Thioketenes are organosulfur compounds analogous to ketenes with the general formula R2C=C=S, where R is alkyl or aryl. They are generally unstable and difficult to prepare. Thioketene (ethenthione) is also the name of the compound CH2=C=S, which is the simplest thioketene. Formula: C2H2S and Molecular weight: 58.102.
Thioketenes are reactive, tending to polymerize. Some thioketenes are produced as transient species upon pyrolysis of 1,2,3-thiadiazoles. They have a nonstabilized Cdouble bondS group and consequently, except for sterically hindered examples, exhibit a high tendency to oligo- or polymerize. Their application in synthesis depends on the availability of convenient routes to generate these species and, at least for the more elusive examples. They are highly electrophilic and give a smooth reaction with ammonia or amines. In fact, addition of a secondary amine offers the best way to scavenge a suspected thioketene.
Bis(trifluoromethyl)thioketene ((CF3)2C=C=S) is a rare example of a stable thioketene. Another stable thioketene is carbon subsulfide (S=C=C=C=S). Recently, a number of routes to thioketenes have been developed, allowing them now to be considered as useful reagents. In the reaction of alkynyl thiolates with excess diethylamine, the amine provides the proton to generate an aldothioketene and, at the same time, serves as the trapping reagent. A marked change in the methylene group geometry between thioketene and ketene is observed, such that a considerable increase in the CH bond length and concomitant decrease in the HCH angle occurs on sulfur substitution.