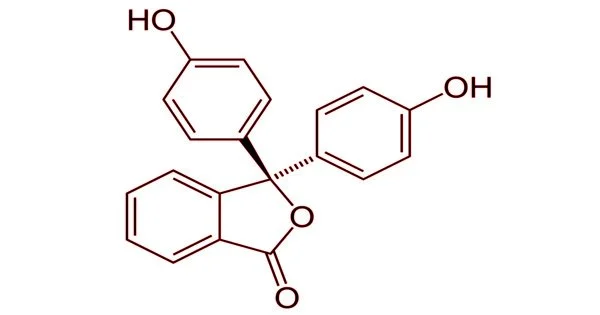
Phenolphthalein is an organic compound that is used as a cathartic in medicine. It is a chemical compound with the formula C20H14O4 and is frequently abbreviated as “HIn”, “HPh”, “phph”, or simply “Ph”. In acid-base titrations, phenolphthalein is frequently used as an indicator. It turns colorless in acidic solutions and pink in basic solutions for this application. It is a type of dye known as a phthalein dye.
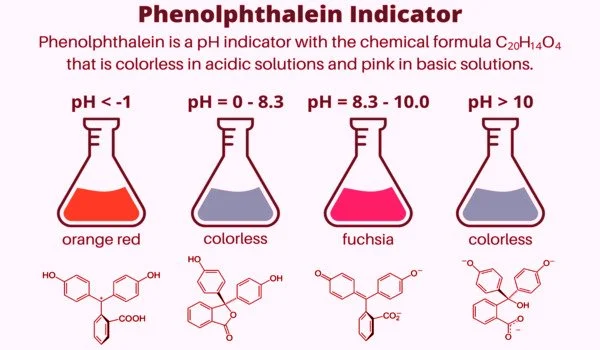
Phenolphthalein is only slightly soluble in water and is usually dissolved in alcohol before being used in experiments. It is a weak acid capable of losing H+ ions in solution. The molecule of nonionized phenolphthalein is colorless, while the ion of double deprotonated phenolphthalein is fuchsia. Further loss of proton in higher pH occurs slowly and leads to a colorless form. Phenolphthalein ion in concentrated sulfuric acid is orange red due to sulfonation.
As an indicator, it is used in acid-base titration. It turns pink to red in alkaline solutions and is colorless in acid solutions. For experimentation purposes, it is dissolved in alcohol and is slightly soluble in water.
Properties
It is both yellowish-white to pale orange or white fine crystalline powder, and it appears colorless in liquid form until PH 8.5, at which point it turns pink to deep red. It has no taste or odor. This chemical compound is commonly used as a PH indicator and as a laboratory agent. Phenolphthalein is produced. It is a weak acid that belongs to the Pthalein dye family.
- Molecular Weight/ Molar Mass: 318.32 g/mol
- Density: 1.277 g/cm3
- Appears: White powder
- Melting Point: 258–263 °C
Phenolphthalein is a colourless, weak acid that is widely used as an indicator in titration experiments to indicate the endpoint of the titration. The endpoint is indicated by the formation of a pink colour since this compound dissociates to form pink anions when dissolved in water.
Uses
pH indicator
The most common application of phenolphthalein is as an indicator in acid-base titrations. It is also a component of universal indicator, alongside methyl red, bromothymol blue, and thymol blue.
Depending on the pH of the solution, phenolphthalein takes on different forms in aqueous solution. In the literature, there is inconsistency regarding the hydrated forms of the compounds and the color of sulfuric acid. Wittke discovered in 1983 that it exists in protonated form (HIn+) under strongly acidic conditions, giving it an orange color. However, a subsequent paper suggested that the color was caused by sulfonation to phenolsulfonphthalein.
The lactone form (HIn) is colorless when exposed to strongly acidic or slightly basic conditions. The pink color comes from the doubly deprotonated (In2-) phenolate form (the anion form of phenol). In strongly basic solutions, phenolphthalein is converted to its In(OH)3 form, and its pink color fades slowly and completely when the pH exceeds 13.
Applications in medicine
Phenolphthalein has been used as a laxative for over a century, but it is now being phased out of over-the-counter laxatives due to concerns about carcinogenicity. Laxatives that used to contain phenolphalein have frequently been reformulated with different active ingredients: Ex-Lax was switched to a senna extract, and Feen-a-Mint was switched to bisacodyl.
Thymolphthalein is a related laxative derived from thymol.
Despite concerns about its carcinogenicity, using phenolphthalein as a laxative is unlikely to cause ovarian cancer. Phenolphthalein has been shown to inhibit human cellular calcium influx via store-operated calcium entry (SOCE, see Calcium release-activated channel Structure). This is achieved by inhibiting thrombin and thapsigargin, two SOCE activators that increase intracellular free calcium.