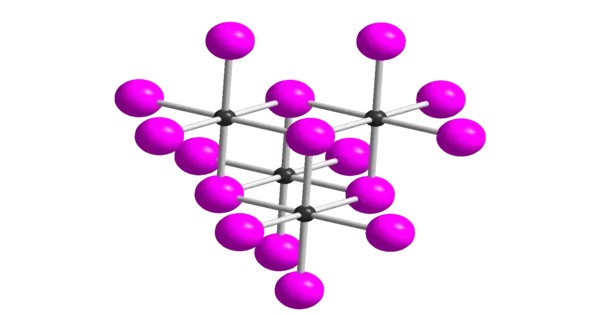
Tellurium tetraazide is an inorganic chemical compound with the formula Te(N3)4. It’s an interesting and relatively rare compound, primarily studied in the context of its chemical properties and potential reactivity. It is a highly sensitive explosive and takes the form of a yellow solid. It has been prepared directly as a precipitate of the reaction between tellurium tetrafluoride and trimethylsilyl azide. It is notable primarily for its high reactivity and explosive nature.
Properties
- Chemical formula: Te(N3)4
- Molar mass: 295.7 g/mol
- Appearance: Yellow solid
- Physical Appearance: It is typically a yellow or orange crystalline solid.
- Solubility: Like many azides, it is poorly soluble in water and may react with moisture to form other compounds.
- Oxidizing Properties: The azide groups are known to be strong oxidizers, which adds to the compound’s explosive potential.
Reactivity
- Instability: Te(N₃)₄ is highly reactive and potentially explosive, especially when subjected to heat or mechanical shock. The azide groups themselves are sensitive and can easily decompose, making the compound hazardous to handle.
- Oxidizing agent: The compound could act as an oxidizing agent due to the presence of the azide groups, which are energetically unstable and could release nitrogen gas upon decomposition.
- Handling precautions: Special care is needed in handling Te(N₃)₄, as its instability and sensitivity to various conditions can lead to violent reactions.
Occurrences
Tellurium Tetraazide is not typically found in nature due to its instability. It is primarily synthesized in laboratories for research purposes, particularly in the study of energetic materials and azide chemistry.
Laboratory Synthesis: It is usually synthesized by reacting tellurium compounds, such as tellurium tetrachloride (TeCl₄), with sodium azide (NaN₃) under controlled conditions.
Safety Concerns
Due to its explosive nature, handling of tellurium tetraazide requires significant safety precautions. It is not a compound that is commonly encountered outside of highly controlled experimental settings.