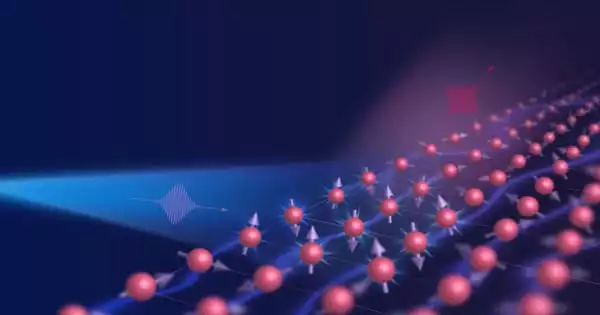
Supercrystals are a specific arrangement of atoms or molecules in a crystalline structure that exhibits distinct and enhanced properties when compared to individual crystals. If there have been advancements in the use of supercrystals for solar energy harvesting, it is likely that they have involved improvements in the efficiency of solar cells or other related technologies.
Hydrogen is a key component of the energy transition. LMU researchers created new high-performance nanostructures to achieve this using solar energy. The material holds the world record for solar-powered green hydrogen production.
When Emiliano Cortés goes in search of sunlight, he does not use massive mirrors or solar farms. On the contrary, the LMU professor of experimental physics and energy conversion is fascinated by the nanocosmos. “Where the high-energy particles of sunlight meet atomic structures is where our research begins,” Cortés said. “We are working on material solutions to use solar energy more efficiently.” His discoveries have enormous potential because they enable novel solar cells and photocatalysts. Cortés is aware of one major challenge: “Sunlight arrives on Earth ‘diluted,’ so the energy per area is comparatively low.”
Where the high-energy particles of sunlight meet atomic structures is where our research begins. We are working on material solutions to use solar energy more efficiently.
Emiliano Cortés
Solar panels make up for this by covering a large area. Cortés, on the other hand, approaches the problem from the opposite side: He is developing plasmonic nanostructures that can be used to concentrate solar energy with his team at LMU’s Nano-Institute, which is funded by the e-conversion cluster of excellence, Solar Technologies go Hybrid (a Bayerisches Staatsministerium für Wissenschaft und Kunst initiative), and the European Research Council.
In a publication in the journal Nature Catalysis, Cortés, together with Dr. Matías Herran and cooperation partners from the Free University of Berlin and the University of Hamburg, present a two-dimensional supercrystal that generates hydrogen from formic acid with the help of sunlight. “The material is so outstanding, in fact, that it holds the world record for producing hydrogen using sunlight,” Cortés points out.

Nano hotspots unleash catalytic power
Cortés and Herrán create their supercrystal using two nanoscale metals. “We first create particles in the range of 10-200 nanometers from a plasmonic metal — which in our case is gold,” Herrán goes on to explain. “At this scale, visible light interacts very strongly with the electrons of gold, causing them to oscillate resonantly.” This allows the nanoparticles to capture more sunlight and convert it into electrons with extremely high energy.
“Highly localized and strong electric fields occur, the hotspots,” says Herrán. These form between the gold particles, which gave Cortés and Herrán the idea of placing platinum nanoparticles right in the interspaces. In the hotspots, we can enable it to convert formic acid into hydrogen,” Herrán explains. With a hydrogen production rate from formic acid of 139 millimoles per hour and per gram of catalyst, the photocatalytic material currently holds the world record for H2 production with sunlight.
An impetus for greener hydrogen production
Today, hydrogen is primarily derived from fossil fuels, primarily natural gas. “By combining plasmonic and catalytic metals, we are advancing the development of potent photocatalysts for industrial applications, such as the conversion of CO2 into usable substances,” the researchers say. Their material development has already been patented by the two researchers.