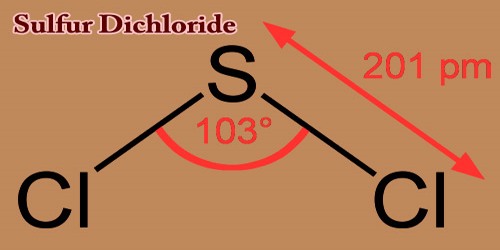
Sulfur dichloride is a fuming liquid, reddish-brown; pungent odor of chlorine, with the formula SCl2. This cherry-red liquid is the simplest and one of the most common sulfur chlorides and is used as a precursor to organosulfur compounds. Decomposes in alcohol and water; is soluble in benzene.
Sulfur dichloride is miscible with several covalent inorganics and organic compounds such as liquid SO2, S2Cl2, SOCl2, SO2Cl2, chlorohydrocarbons, and benzene. This is created by either elemental sulfur or disulfur dichloride chlorination. This method takes place in a sequence of steps, some of which are:
S8 + 4 Cl2 → 4 S2Cl2; ΔH = −58.2 kJ/mol
S2Cl2 + Cl2 ↔ 2 SCl2; ΔH = −40.6 kJ/mol
Sulfur dichloride utilized as chlorine transporter or chlorinating operator, elastic vulcanizing, vulcanized oils, decontaminating sugar juices, a sulfur dissolvable, chloridizing specialist in metallurgy, assembling of natural synthetics, and bug sprays. Adding Cl2 to S2Cl2 was proposed to proceed via a mixed-valence Cl3S-SCl intermediate. SCl2 is further chlorinated to give SCl4 but this species is unstable at room temperature. It is likely that several SxCl2 exist where x > 2.
Preparation of sulfur dichloride:
Fifty grams of sulfur monochloride are put in the refining carafe. About 0.1 g of iron powder is added to the fluid as a halogen transporter and dry chlorine is driven inconsistently (3-4 air pockets/sec) for one-half hour. The dull fluid that is delivered is permitted to stand one hour and afterward one milliliter of phosphorus trichloride is included. The product is fractionated through a little segment stuffed with glass and the bit that bubbles at 55-62°C are gathered. This division is redistilled as before from a couple of drops of phosphorus trichloride and the unadulterated sulfur dichloride is gathered at 59-61°C. Yield about 55 g. In the presence of a trace of phosphorus tri-chloride, the deep-red content is stable at room temperature for several days; it then gradually decomposes into chlorine and sulfur monochloride and can be re-purified as defined by distillation.
Aldehydes and epoxides within the sight of hydrochloric corrosive reason rough polymerization. Liquor and glycols within the sight of hydrochloric corrosive lead to drying out responses. Toxin aggravation and destructive to shin, eyes, and mucous layers. Unadulterated examples might be put away in fixed glass ampules which build up a slight positive weight of chlorine, ending the decay. When heated to decomposition it emits very toxic fumes of SOx and Cl-.
The SCl2 must be applied at room temperature as quickly as possible after distillation within one hour. It is used in organic synthesis and adds chloride-substituted thioethers to alkenes. With water, the dichloride structures sulfuric corrosive, sulfur, and a blend of thionic acids, H2SxOy. Sulfur dichloride solvent in hexane, carbon tetrachloride, carbon disulfide, and ethylene dichloride.
Information Sources: