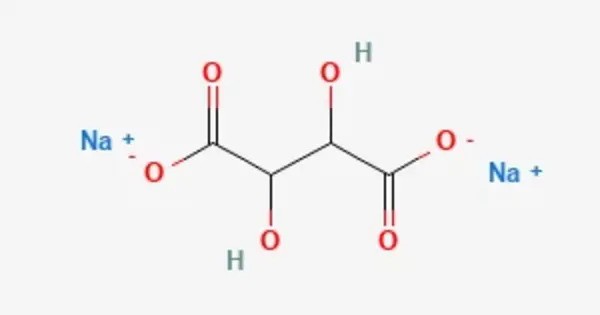
Sodium tartrate (Na2C4H4O6) is a salt used as an emulsifier and a binding agent in food products such as jellies, margarine, and sausage casings. It typically occurs as a crystalline solid that is highly soluble in water. It is non-volatile, stable under normal conditions, and has a slightly saline taste. It is the sodium salt of tartaric acid, an organic compound widely used in food, pharmaceutical, and laboratory applications. As a food additive, it is known by the E number E335.
It is made by the combination reaction of baking soda/Sodium Bicarbonate (NaHCO₃) with tartaric acid. Because its crystal structure captures a very precise amount of water, it is also a common primary standard for Karl Fischer titration, a common technique to assay water content.
Properties
- Chemical formula: C4H4Na2O6 (anhydrous), C4H8Na2O8 (dihydrate)
- Molar mass: 194.051 g/mol (anhydrous), 230.082 g/mol (dihydrate)
- Appearance: white crystals
- Density: 1.545 g/cm3 (dihydrate)
- Solubility in water: soluble
- Solubility: insoluble in ethanol
Occurrences
Sodium tartrate is the sodium salt of tartaric acid, a naturally occurring organic acid found in many plants, particularly in grapes, tamarinds, bananas, and citrus fruits. Industrially, it is often obtained as a byproduct in wine production, where tartaric acid is extracted and neutralized with sodium compounds.
It occurs most commonly in its dihydrate form and is widely used as a food additive (E335), a laboratory reagent in Fehling’s solution (for reducing sugar tests), and sometimes in pharmaceuticals as a mild laxative.
Application
In the food industry, sodium tartrate acts as an emulsifier, buffering agent, and acidity regulator, commonly designated as E335. It helps maintain the pH of foods and beverages and prevents crystallization in sugar syrups. Because it is safe for consumption in controlled amounts, it is often added to jams, jellies, soft drinks, and confectioneries.
In pharmaceuticals, sodium tartrate is sometimes used as a mild laxative due to its osmotic properties, drawing water into the intestines. It can also be employed in the preparation of effervescent tablets and other medicinal formulations.
In laboratories, sodium tartrate serves as a primary standard in chemical analysis, especially in the standardization of Karl Fischer reagent for water content determination. Its stable crystalline form and known purity make it reliable for precise measurements.