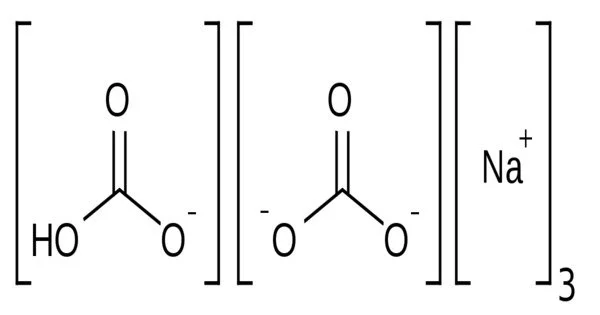
Sodium sesquicarbonate Na3H(CO3)2 is a double salt of sodium bicarbonate and sodium carbonate (NaHCO3 · Na2CO3), and has a needle-like crystal structure. However, the term is also applied to an equimolar mixture of those two salts, with whatever water of hydration the sodium carbonate includes, supplied as a powder.
The dihydrate, Na3H(CO3)2 · 2H2O, occurs in nature as the evaporite mineral trona.
Due to concerns about the toxicity of borax which was withdrawn as a cleaning and laundry product, sodium sesquicarbonate is sold in the European Union (EU) as “Borax substitute”. It is also known as one of the E number food additives E500(iii).
Properties
Sodium sesquicarbonate typically appears as white or colorless crystals or as a white powder. It has a mild alkaline taste. It forms in alkaline lake environments where evaporation and subsequent chemical reactions occur over time. It is often found alongside other evaporite minerals such as halite (common salt) and gypsum.
- Chemical formula: Na3H(CO3)2·2H2O
- Appearance: white, needle-like
- Density: 2.112 g/cm3 (dihydrate)
- Solubility in water: dehydrate [13 g/100 mL (0 °C); 42 g/100 mL (100 °C)]
- Solubility: It is moderately soluble in water, with a solubility of about 16.4 grams per 100 milliliters of water at 20°C. The solubility increases with higher temperatures.
- pH: It is alkaline in nature and has a pH of around 9-11 in aqueous solutions. It can act as a buffering agent to maintain the pH of a solution.
- Decomposition: When heated, releasing water and carbon dioxide. The decomposition temperature is approximately 34-38°C (93-100°F).
Uses
Sodium sesquicarbonate is used in bath salts, swimming pools, as an alkalinity source for water treatment, and as a phosphate-free product replacing the trisodium phosphate for heavy duty cleaning.
- Manufacturing of glass: It is used as a source of sodium carbonate in the production of glass, where it acts as a flux to lower the melting point of silica.
- Water treatment: It can be used in water treatment processes to adjust pH levels and remove impurities.
- Cleaning agent: It is found in some cleaning products as a mild abrasive and alkaline cleaner. It can help remove stains and odors.
- pH regulation: It can be utilized to raise the pH levels in swimming pools and spas.
Safety
Sodium sesquicarbonate is generally considered safe for handling and use. However, like any chemical substance, it is important to follow proper safety precautions and guidelines when working with it.