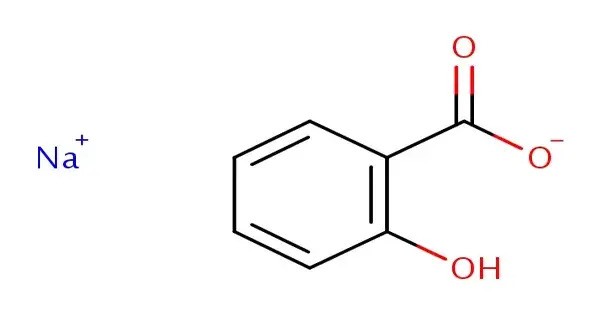
Sodium salicylate is a sodium salt of salicylic acid. It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure. It is a white, crystalline powder that is soluble in water and alcohol. As an important derivative of salicylic acid, it has long been used for its analgesic, antipyretic, and anti-inflammatory properties. It was one of the earliest synthetic alternatives to natural salicylates derived from willow bark and predates the widespread use of aspirin (acetylsalicylic acid). Historically, it has been synthesized by refluxing methyl salicylate (wintergreen oil) with an excess of sodium hydroxide.
In medicine, sodium salicylate acts by inhibiting cyclooxygenase (COX) enzymes, thereby reducing the synthesis of prostaglandins that cause pain, fever, and inflammation. Unlike aspirin, it does not irreversibly acetylate COX, making it milder on platelets and sometimes preferred for patients who cannot tolerate aspirin. It has been used in treating rheumatic fever, arthritis, and mild pain relief, though its use has declined with modern nonsteroidal anti-inflammatory drugs (NSAIDs).
Properties
Sodium salicylate is of the salicylate family. It is a shiny white powder with an aromatic flavor. It has a molecular weight of 160.10 g/mol, and can produce mildly alkaline solutions because it can donate 1 hydrogen bond and accept 3. It exhibits analgesic, antipyretic, and anti-inflammatory properties, making it one of the earliest synthetic drugs developed for medical use.
- Chemical formula: C7H5NaO3
- Molar mass: 160.104 g/mol
- Appearance: White crystals
- Melting point: 200 °C (392 °F; 473 K)
- Solubility in water: 25.08 g/100 g (-1.5 °C), 107.9 g/100 g (15 °C), 124.6 g/100 g (25 °C), 179 g/100 g (114 °C)
- Solubility: Soluble in glycerol, 1,4-Dioxane, alcohol
- Solubility in methanol: 26.28 g/100 g (15 °C), 34.73 g/100 g (67.2 °C)
Synthesis
Sodium salicylate can be prepared by neutralizing salicylic acid with a sodium base such as sodium hydroxide or sodium carbonate or by refluxing methyl salicylate with sodium hydroxide. It has a slightly sweetish, saline taste. The compound is stable under normal conditions but should be kept in dry, sealed containers to prevent degradation.
Occurrences
Sodium salicylate is not widely found in nature in its salt form but is derived from salicylic acid, which naturally occurs in plants like willow bark, meadowsweet, and other members of the Salicaceae family. Historically, extracts of willow bark containing salicin (a salicylate glycoside) were used for pain relief and fever reduction. Industrially, sodium salicylate is produced synthetically by neutralizing salicylic acid with sodium hydroxide. Today, it is used in medicine, dye manufacturing, preservatives, and as a starting material in organic synthesis.
Uses
It is used in medicine as an analgesic and antipyretic. It also acts as non-steroidal anti-inflammatory drug (NSAID), and induces apoptosis in cancer cells and also necrosis. It is also a potential replacement for aspirin for people sensitive to it. It may also be used as a phosphor for the detection of vacuum ultraviolet radiation and beta radiation.
Beyond medicine, sodium salicylate finds applications in fluorescence studies, biochemistry, and material science, as it exhibits photoluminescent properties. It is also employed as a preservative and intermediate in chemical synthesis.