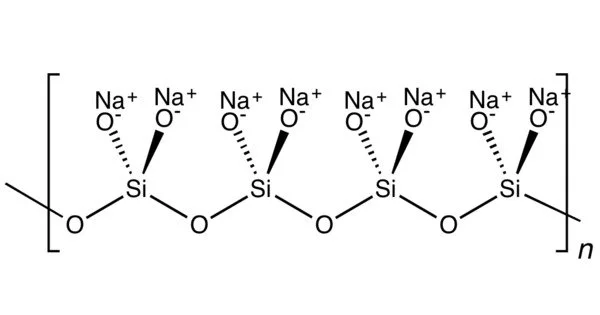
The main component of commercial sodium silicate solutions is sodium metasilicate, which has the chemical formula Na2SiO3. It is a silicate compound that is commonly referred to as water glass or liquid glass. In a high-temperature fusion process, sodium carbonate (soda ash) reacts with silica sand to produce sodium metasilicate. It comes in a variety of hydrated forms.
It is an ionic compound consisting of sodium cations Na+ and the polymeric metasilicate anions [–SiO2−3–]n. It is a colorless crystalline hygroscopic and deliquescent solid, soluble in water (giving an alkaline solution) but not in alcohols.
Properties
- Chemical formula: Na2SiO3
- Molar mass: 122.062 g·mol−1
- Appearance: White crystals
- Density: 2.61 g/cm3
- Melting point: 1,088 °C (1,990 °F; 1,361 K)
- Solubility in water: 22.2 g/100 ml (25 °C); 160.6 g/100 ml (80 °C)
- Solubility: insoluble in alcohol
- Refractive index (nD): 1.52
Preparation and properties
The anhydrous compound can be prepared by fusing silicon dioxide SiO2 (silica, quartz) with sodium oxide Na2O in 1:1 molar ratio.
The compound crystallizes from solution as various hydrates, such as –
- pentahydrate Na2SiO3·5H2O (CAS 10213-79-3, EC 229-912-9, PubChem 57652358)
- nonahydrate Na2SiO3·9H2O (CAS 13517-24-3, EC 229-912-9, PubChem 57654617)
Structure
In the anhydrous solid, the metasilicate anion is actually polymeric, consisting of corner-shared {SiO4} tetrahedra, and not a discrete SiO32− ion.
In addition to the anhydrous form, there are hydrates with the formula Na2SiO3·nH2O (where n = 5, 6, 8, 9), which contain the discrete, approximately tetrahedral anion SiO2(OH)22− with water of hydration. For example, the commercially available sodium silicate pentahydrate Na2SiO3·5H2O is formulated as Na2SiO2(OH)2·4H2O, and the nonahydrate Na2SiO3·9H2O is formulated as Na2SiO2(OH)2·8H2O. The pentahydrate and nonahydrate forms have their own CAS Numbers, 10213-79-3 and 13517-24-3 respectively.
Applications
Because of its strong alkalinity and ability to break down grease, oils, and stains, sodium metasilicate is a key ingredient in many detergents and cleaning agents. Laundry detergents, dishwasher detergents, and all-purpose cleaners are all common sources of it.
It is used as an additive in cement and concrete formulations to improve water and chemical resistance. It’s used as a binder in a variety of applications, including refractory materials, to keep the components together. It is occasionally used as a fireproofing agent in certain materials. It is used in water treatment processes to prevent corrosion and control scale formation in boilers and other water systems.