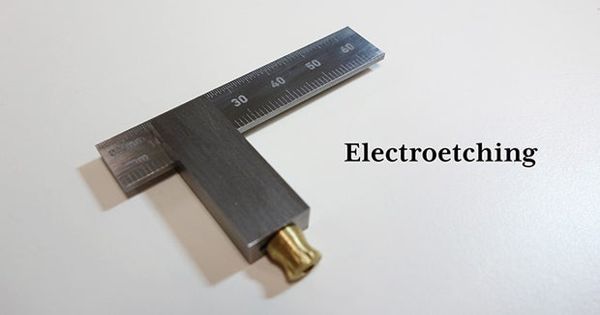
Electroetching is a simple metal etching process. It is a metal etching process that involves the use of a solution of an electrolyte, an anode, and a cathode. It involves a salt solution and an electric current. It is the use of electrolysis for the removal of selected areas of a metal surface.
It is a mode of etching upon metals by electrolytic action. The metal piece to be etched is connected to the positive pole of a source of direct electric current. It involves a salt solution and an electric current. A piece of the same metal is connected to the negative pole of the direct current source and is called the cathode. The negative is attached to a swab.
In order to reduce unwanted electrochemical effects, the anode and the cathode should be of the same metal. When electroetching, simply press the wet swab against the steel you want marked. You will notice the water on the swab will start to sizzle and turn black as it etches. Similarly the cation of the electrolyte should be of the same metal as well. The higher voltage you use, the faster it goes.
The etching method comprises the following steps: arranging a mask on the surface of the amorphous alloy member; carrying out chemical etching on the amorphous alloy member provided with the mask on its surface by using a chemical etching solution; carrying out electroetching on the amorphous alloy member having undergone chemical etching by using the electroetching solution, and removing the mask on the surface of the amorphous alloy member after electroetching so as to obtain the amorphous alloy member with an etched pattern. When the current source is turned on, the metal of the anode is dissolved and converted into the same cation as in the electrolyte and at the same time, an equal amount of the cation in the solution is converted into metal and deposited on the cathode.
Depending on the voltage used and the concentration of the electrolyte, other, more complex electrochemical effects can take place at the anode and the cathode but the solution at the anode and deposition at the cathode are the main effects. After etching you have to clean your blade, and remove the masking you used. Make sure all the salt water is out of the etched pattern.
Information Source: