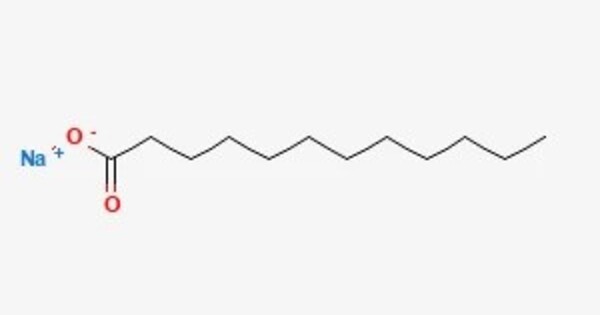
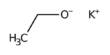
Sodium laurate is a chemical compound with formula CH3(CH2)10CO2Na. As the sodium salt of a fatty acid (lauric acid), it is classified as a soap. It is a white solid. It is a sodium salt of lauric acid, which is a fatty acid derived from coconut oil or palm kernel oil.
It’s commonly used in various personal care products like soaps, shampoos, and cleansers due to its surfactant and emulsifying properties. In these products, sodium laurate helps to create lather and improve the texture and stability of formulations.
Properties
Typically, it appears as a white or off-white powder. It is soluble in water, especially in hot water, due to its ionic nature. In solution, it can be mildly alkaline. It is generally stable under normal conditions. However, it can hydrolyze in very acidic or basic conditions.
- Chemical formula: C12H23NaO2
- Molar mass: 222.304 g·mol−1
- Density: 1.102 g/ml
- Melting point: 244 to 246 °C (471 to 475 °F; 517 to 519 K)
Natural Sources
Sodium laurate is not commonly found in its pure form in nature but is derived from lauric acid, which is a naturally occurring fatty acid. Lauric acid is found in various natural fats and oils, particularly in coconut oil and palm kernel oil.
Industrial Production
Sodium laurate is produced by saponifying lauric acid (typically obtained from coconut oil or palm kernel oil) with sodium hydroxide. This process creates sodium laurate and water.
Use
Sodium laurate is frequently used in bars of soap as an ingredient. Sodium laurate is also a permitted bleaching, washing and peeling agent. It has also been used to induce peripheral arterial disease in rats.
- Soaps and Detergents: It is used as a surfactant, helping to create foam and improve cleaning.
- Cosmetics: It can be found in shampoos, body washes, and other personal care products.
- Pharmaceuticals: It may be used as an emulsifier or in the formulation of certain medicines.