Spectrophotometry is a subfield of electromagnetic spectroscopy that deals with the quantitative measurement of a material’s reflection or transmission properties as a function of wavelength. It is a technique for determining the intensity of light at various wavelengths in a spectrum. This technique is widely used in chemistry, biochemistry, physics, environmental science, and biology, among other scientific and analytical fields.
Basic Principle
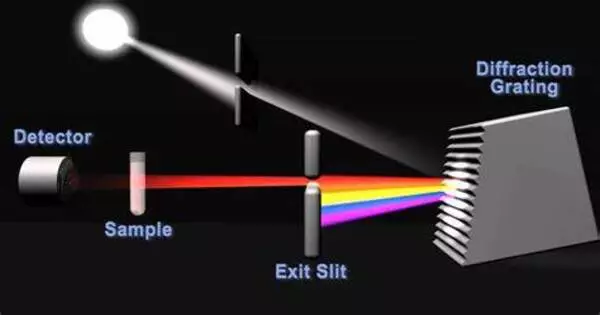
The fundamental principle of spectrophotometry is to pass a light beam through a sample and measure how much light is absorbed or transmitted at different wavelengths. A spectrophotometer is the instrument used for this purpose.
Spectrophotometry employs photometers called spectrophotometers, which can measure the intensity of a light beam at various wavelengths. Although ultraviolet, visible, and infrared radiation are the most commonly used wavelengths for spectrophotometry, modern spectrophotometers can probe a wide range of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.
Types
There are various types of spectrophotometers, but they generally share some common components:
- Light Source: Light of various wavelengths is emitted by a light source. Tungsten lamps, deuterium lamps, and xenon lamps are common light sources.
- Monochromator: This component chooses a particular wavelength of light from the light source. A prism or diffraction grating can be used to disperse light into its individual wavelengths.
- Sample Holder: A sample holder, also known as a cuvette, is a container that holds the sample and allows light to pass through it. Depending on its composition, the sample may absorb some of the light.
- Detector: The detector detects the amount of light passing through the sample. Photodiodes and photomultiplier tubes are common detectors.
- Amplifier and Readout: The detector’s signal is amplified and converted into a readable output, which is typically displayed on a screen or recorded electronically.
Applications
- Quantitative Analysis: It can be used to determine the concentration of a substance in a sample by measuring the amount of light absorbed or transmitted. This is based on the Beer-Lambert Law, which relates absorbance to concentration.
- Qualitative Analysis: It can be used to identify substances based on their unique absorption or transmission spectra.
- Kinetic Studies: It can be used to study chemical reactions over time by monitoring changes in absorbance.
- Biological and Biochemical Applications: It is widely used in fields such as biochemistry and molecular biology to analyze nucleic acids, proteins, and other biomolecules.
Spectrophotometry is a versatile and powerful analytical technique, and its applications span a wide range of scientific disciplines.