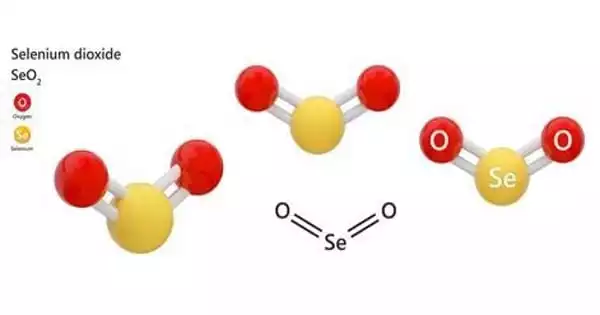
The chemical compound with the formula SeO2 is selenium dioxide. It has a pungent sour odor and looks like a white or creamy-white volatile lustrous crystal or crystalline powder. This white solid is one of the most commonly seen selenium compounds.
When heated to roughly 350°C, selenium dioxide sublimes to generate a greenish-yellow vapor with a distinct odor that is typically described as unpleasant and smelling of rotting horseradish. When dissolved in water, it produces selenous acid, which is commonly utilized in processes.
Properties
It comes in the form of a yellowish-white to reddish powder or crystals. It is water-soluble and hygroscopic, absorbing moisture or water from the air. Strong oxidizing agents, reducing agents, strong acids, ammonia, organics, and phosphorus trichloride are all incompatible with selenium oxide.
Solid SeO2 is a one-dimensional polymer with an alternating chain of selenium and oxygen atoms. Each Se atom has a pyramidal structure and a terminal oxide group. The lengths of the bridging Se-O bonds are 179 pm, and the terminal Se-O distance is 162 pm. Along the polymer chain, the relative stereochemistry at Se alternates (syndiotactic).
- Melting point: 315 °C (subl.)(lit.)
- Boiling point: 684.9 °C(lit.)
- Density: 4.81 g/mL at 25 °C(lit.)
- vapor pressure: 1 mm Hg ( 157 °C)
- Flash point: 315°C
- storage temp.: Store below +30°C.
- solubility H2O: soluble
- Form: powder
- Color: white
- Specific Gravity: 3.95
SeO2 is an acidic oxide because it dissolves in water to generate selenous acid. The phrases selenous acid and selenium dioxide are frequently used interchangeably. It interacts with bases to generate selenite salts containing the anion SeO32-. For instance, a reaction with sodium hydroxide yields sodium selenite:
SeO2 + 2 NaOH → Na2SeO3 + H2O

Preparation
Selenium dioxide is prepared by oxidation of selenium by burning in the air or by reaction with nitric acid or hydrogen peroxide, but perhaps the most convenient preparation is by the dehydration of selenous acid.
2 H2O2 + Se → SeO2 + 2 H2O
3 Se + 4 HNO3 + H2O → 3 H2SeO3 + 4 NO
H2SeO3 ⇌ SeO2 + H2O
Selenium dioxide is obtained by burning selenium metal in oxygen:
Se + O2 → SeO2
Selenium also forms a trioxide, SeO3. In excess of oxygen, the product mixture may contain both dioxide and trioxide. The trioxide is unstable.
Selenium dioxide may be prepared by heating selenium with oxygen and nitrogen dioxide. The presence of excess oxygen would oxidize nitrogen dioxide to pentoxide, instead of converting selenium dioxide to trioxide:
2Se + 3O2 + 4NO2 → 2SeO2 + 2N2O5
Selenium dioxide also may be produced by the oxidation of selenium by nitric acid. The overall reaction may be written as follows:
Se + 2HNO3 → SeO2 + H2O + NO2 + NO
Application
It is utilized in the oxidation of a variety of organic compounds, including the conversion of paraldehyde – a triplet of acetaldehyde molecules connected in a cyclic configuration – to glyoxal, which is practically the simple alkane ethane with four hydrogen atoms replaced by two oxygens. This is beneficial since glyoxal is an excellent building block for chemical synthesis.
Occurrence
Downeyite, a natural form of selenium dioxide, is a highly rare mineral. It is only discovered in a few smoldering coal wastes.