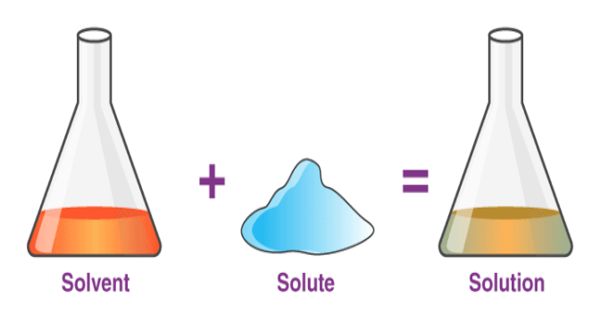
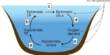
A solvent is the component of a solution that is present in the greatest amount. It is a substance that becomes a solution by dissolving a solid, liquid, or gaseous solute. It is the liquid in which a solute is dissolved to form a solution. A solvent is usually a liquid, but can also be a solid or gas. The amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample. The most common solvent in everyday life is water.
The solvent in chemistry is a substance in which solute is dissolved and forms solution is a solvent. These can be categorized as organic or inorganic, and in terms of chemical polarity.
Most other commonly-used solvents are organic (carbon-containing) chemicals. These may be predominantly acidic, predominantly basic, amphoteric (both), or aprotic (neither). These are called organic solvents. Solvents usually have a low boiling point and evaporate easily or can be removed by distillation, thereby leaving the dissolved substance behind. Solvents that contain carbon and hydrogen only are hydrocarbon solvents such as gasoline, benzene, toluene, hexane, etc.
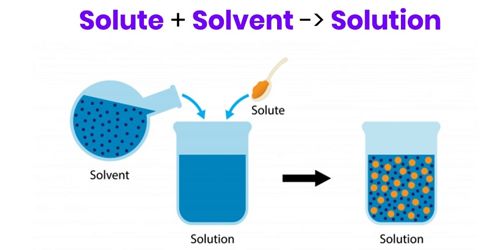
Solvents are usually clear and colorless liquids and many have a characteristic smell. The concentration of a solution is the amount of compound that is dissolved in a certain volume of solvent. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. Solubility is the maximal amount of compound that is soluble in a certain volume of solvent at a specified temperature. Eventually, the molecules of solute become evenly distributed throughout the solvent.
Many different solvents are used in a wide variety of everyday product applications – from paint, personal care products and pharmaceuticals, to pesticides, cleaners and inks. The uses of solvents may be the following: Paints and coatings, Inks, Personal care products such as cosmetics, nail remover, Cleaning products, Healthcare applications such as pharmaceutical products, etc. Without solvents, many products we rely on would not perform as well.
Common uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene), as paint thinners (e.g. toluene, turpentine), as nail polish removers and glue solvents (acetone, methyl acetate, ethyl acetate), in spot removers (e.g. hexane, petrol ether), in detergents (citrus terpenes), in perfumes (ethanol), and in chemical syntheses. Inorganic solvents are used in research chemistry and in a few technological processes.
Information Source: