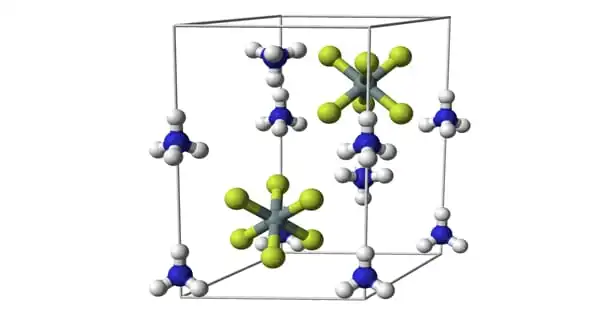
The formula for ammonium fluorosilicate is (NH4)2SiF6. It has the appearance of a white crystalline solid. It is a hazardous compound, as are all fluorosilicic acid salts. It is composed of white crystals with at least three polymorphs and can be found in nature as the rare minerals cryptohalite or bararite. It’s utilized as a disinfectant, as well as in glass etching, metal casting, and electroplating.
Hydrofluoric acid, a source of fluoride ions, is formed when ammonium fluorosilicate combines with water. The fluoride ion, unlike other halide ions, is very reactive, acting as a weak base and partaking in certain unusual reactions. Fluorides, in particular, react strongly with molecules containing calcium, magnesium, or silicon ions, which implies that soluble fluoride solutions are corrosive to both living tissue and glass. Hydrofluoric acid can cause severe chemical burns and is one of the few materials that can etch glass. It is also a toxic gas in its anhydrous form.
Properties
- Appearance: White Crystals
- Density: 2.0 g/cm-3
- Melting Point: 100 °C
- Molar Mass: 178.15 g mol-1
- Molecular Formula: H8F6N2Si
- Solubility: Soluble
Structure
Ammonium fluorosilicate has three major polymorphs: α-(NH4)2[SiF6] form is and corresponds to the mineral cryptohalite. The β form is trigonal (scalenohedral) and occurs in nature as mineral bararite. A third (γ) form was discovered in 2001 and identified with the hexagonal 6mm symmetry. In all three configurations, the [SiF6]2– octahedra are arranged in layers. In the α form, these layers are perpendicular to directions.
In the β- and γ- forms, the layers are perpendicular to the c-axis. The silicon atoms of α- (NH4)2[SiF6] (alpha), have cubic close packing (CCP). The γ form has hexagonal close packing and the β-(NH4)2[SiF6] has primitive hexagonal packing. In all three phases, 12 fluorine atoms neighbor the (NH4)+.

Natural occurrence
This chemical makes rare appearances in nature. It is found as a sublimation product of fumaroles and coal fires. As a mineral, it is either called cryptohalite or bararite, the two being two polymorphs of the compound.
Chemical properties and health hazards
Although ammonium fluorosilicate is noncombustible, it will emit hazardous gases such as hydrogen fluoride, silicon tetrafluoride, and nitrogen oxides in the event of a fire. Aluminium will rust as a result. Ammonium fluorosilicate dissolves in water to generate an acid solution. Inhaling dust can cause lung inflammation and even death. Ingestion can also be deadly. Contact with dust causes inflammation of the eyes, as well as irritation or ulceration of the skin.
Inhaling ammonium fluorosilicate dust can cause lung inflammation and, in extreme cases, death. Ingestion can be deadly. Contact with ammonium fluorosilicate dust produces eye irritation as well as skin irritation or ulceration.
Uses
Ammonium fluorosilicate finds use as a disinfectant, and it is useful in etching glass, metal casting, and electroplating. It is also used to help neutralize washing machine water as laundry sour.