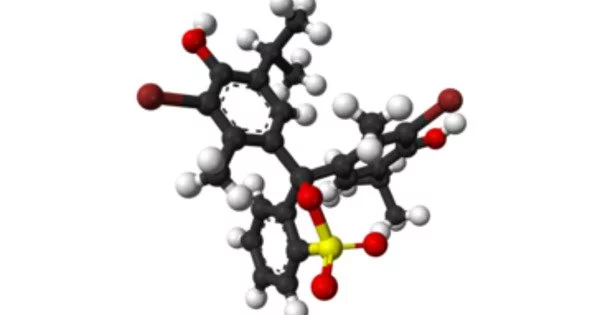
Phenylacetic acid (PAA; conjugate base phenylacetate) is a toluene monocarboxylic acid in which one of the methyl group hydrogens has been substituted by a group of carboxies. This is also known by different synonyms and is a white solid with a heavy honey-like scent. It is a monocarboxylic acid, a benzenes member, and a phenylacetic acid member. Endogeneously, it is a catabolite of phenylalanine.
Phenylacetic acid incorporates a role as a toxin, a personality’s metabolite, an escherichia metabolite, a plant metabolite, a Saccharomyces cerevisiae metabolite, an EC 6.4.1.1 (pyruvate carboxylase) inhibitor, an Aspergillus metabolite, a plant growth retardant, an allergen, and an auxin. As a billboard chemical, because it are often utilized in the illicit production of phenylacetone (used within the manufacture of substituted amphetamines), it’s subject to controls in countries including the United States and China. It derives from an ethanoic acid. it’s a conjugate acid of a phenylacetate.
Phenylacetic acid’s synonyms include α-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid, and β-phenylacetic acid. PAA is extracted into ethylacetate and therefore the extract is mixed with 10% triethylamine in methanol and evaporated to dryness. The presence of triethylamine, by forming a non-volatile salt which doesn’t interfere with the next derivatization of PAA, prevents the evaporation loss of PAA.
Phenylacetic acid is an organic compound which contains a functional group of phenyls and carboxylic acids. It was found to be an active auxin (a form of plant hormone), primarily found in fruit. Nevertheless, its activity is much lower than the activity of indole-3-acettic acid, the essential auxin molecule. It is a solid white, with an unpleasant odor.
In addition, the molecule is of course produced by the metapleural gland of most ant species and used as an antimicrobial. Excess phenylalanine within the body may be disposed of through a transamination process resulting in the assembly of phenylpyruvate. Phenylacetate esterases found within the human liver cytosol. Human plasma esterase also hydrolyzes phenylacetate. Phenylacetate hydrolysis involved arylesterase in plasma, both arylesterase and carboxylesterase in liver microsomes and carboxylesterase in liver cytosol. Plasma hydrolysis is a smaller amount important and overall esterase activity is lower in humans than within the rat.
Phenylacetic acid is a toxic air waste and will not or can only be reached very slowly when this material is evaporated at 20 ° C. It is used in certain perfumes since even in low concentrations it contains a honey-like fragrance. It is also used in the manufacture of penicillin G and of diclofenac.
Phenylacetic acid is additionally employed to treat type II hyperammonemia to assist reduce the amounts of ammonia in a very patient’s bloodstream by forming phenylacetyl-CoA, which then reacts with nitrogen-rich glutamine to make phenylacetylglutamine. This compound is then excreted from the patient’s body. It’s density: 1.033 at 20 °C/4 °C. BP: 226 °C at 760 millimeters of mercury, 135 °C at 32 pressure unit, 121 °C at 20 torr. it is also employed in the illicit production of phenylacetone, which is employed within the manufacture of methamphetamine.
Phenylacetic acid is also used as an adjunctive treatment for treating acute hyperammonemia and associated encephalopathy in patients with urea cycle enzyme deficiencies. The phenylacetic acid sodium salt, sodium phenylacetate, is used as a pharmaceutical drug for the treatment of urea cycle disorders, including as the sodium phenylacetate / sodium benzoate combination (Ammonul). It is used in several drug preparations including camylofin, bendazol, triafungin, phenacemide, lorcainide, phenindione, and cyclopentolate.
Information Sources: