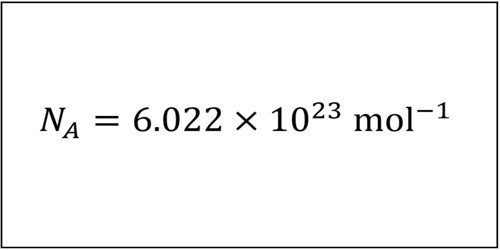Passivation is the process of treating or coating a metal in order to reduce the chemical reactivity of its surface. In physical chemistry and engineering, it refers to a material becoming “passive,” that is, less affected or corroded by the environment of future use. In stainless steel, It means removing the free iron from the surface of the metal using an acid solution to prevent rust. Passivation involves the creation of an outer layer of shield material that is applied as a micro coating, created by chemical reaction with the base material or allowed to build from spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of protective material, such as metal oxide, to create a shell against corrosion. When the surface iron is removed, the other components of the alloy are left behind as a surface layer over the underlying steel.
Passivation can occur only in certain conditions and is used in microelectronics to enhance silicon. The technique of passivation strengthens and preserves the appearance of metallics. In the passivation process, free iron is removed from the surface into a solution, leaving behind a higher chromium level. Through passivation can improve the corrosion resistance of certain stainless steel alloys, it does not eliminate imperfections like micro-cracks, burrs, heat tint, and oxide scale. Other proprietary systems to avoid electrode passivation, several discussed below, are the subject of ongoing research and development.
To understand the passivation of stainless steel, it is critical to look at stainless steel itself. Mechanical finishing of stainless steel surfaces allows for a very smooth surface and is a prerequisite for subsequent electro-polishing. All stainless steels are alloys of iron, nickel, and chromium. Chromium makes up at least 10% of the metal. It is this element that gives stainless steel its resistance to corrosion. When stainless steel parts are manufactured, they can be left with free iron and other foreign material embedded in the surface. Often steel-makers add molybdenum to enhance chromium’s protective characteristics for highly corrosive or high-temperature applications. The possibility of surface contamination getting pushed or smeared into the
A metal surface or sub-surface also exists.
Passivation is a widely-used metal finishing process to prevent corrosion. When exposed to air, many metals naturally form a hard, relatively inert surface, as in the tarnish of silver. In stainless steel, the passivation process uses nitric acid or citric acid to remove free iron from the surface. In the case of other metals, such as iron, a somewhat rough porous coating is formed from loosely adherent corrosion products. In this case, a substantial amount of metal is removed, which is either deposited or dissolved in the environment. The chemical treatment leads to a protective oxide layer that is less likely to chemically react with air and cause corrosion.