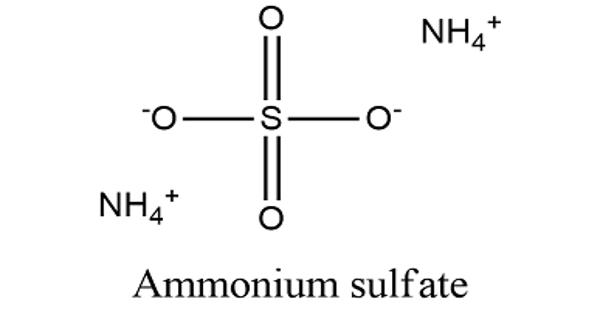
Ammonium sulfate (NH4)2SO4, is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. It is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. Its high solubility provides versatility for a number of agricultural applications. It contains 21% nitrogen and 24% sulfur. It does not dissolve in acetone.
Properties
Ammonium Sulfate is non-hazardous to humans. A high-melting (decomposes above 280℃) white solid which is very soluble in water (70.6 g/100 g water at 0℃; 103.8 g/100 g water at 100℃), it is widely used as a fertilizer for alkaline soils. It has no smell and dissolves in water easily. It has a role as a fertilizer. It is an ammonium salt and an inorganic sulfate salt. It appears as a crystalline solid white and has a salty taste.
- Molecular Weight/ Molar Mass: 132.14 g/mol
- Density: 1.77 g/cm³
- Appears: Fine white crystals or hygroscopic granules
- Melting Point: 235 to 280 °C

Preparation
Ammonium sulfate is made by treating ammonia with sulfuric acid:
2 NH3 + H2SO4 → (NH4)2SO4
A mixture of ammonia gas and water vapor is introduced into a reactor that contains a saturated solution of ammonium sulfate and about 2 to 4% of free sulfuric acid at 60 °C. The reaction between the sulphuric acid and the ammonium hydroxide produces ammonium sulfate. Concentrated sulfuric acid is added to keep the solution acidic, and to retain its level of free acid. The heat of reaction keeps reactor temperature at 60°C. Dry, powdered ammonium sulfate may be formed by spraying sulfuric acid into a reaction chamber filled with ammonia gas. Sulphuric acid is a strong acid and ammonium hydroxide is a weak base. Therefore, the salt formed by ammonium sulphate is acidic. The heat of reaction evaporates all water present in the system, forming a powdery salt.
Ammonium sulfate also is manufactured from gypsum (CaSO4·2H2O). Finely divided gypsum is added to an ammonium carbonate solution. Calcium carbonate precipitates as a solid, leaving ammonium sulfate in the solution.
(NH4)2CO3 + CaSO4 → (NH4)2SO4 + CaCO3
Ammonium sulfate occurs naturally as the rare mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps.
Uses
- The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil, while contributing essential nitrogen for plant growth.
- It is also used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides.
- It is used in the chemical fractionation of proteins.
- It is used in the precipitation of ammonium per sulfate and other ammonium salts.
- It is used as a vaccine to control various diseases, it is used in medicine as it is safe for consumption.
- It is used as a pH controller for solutions and cultures.
- It is used in textile industries.
Information Source: