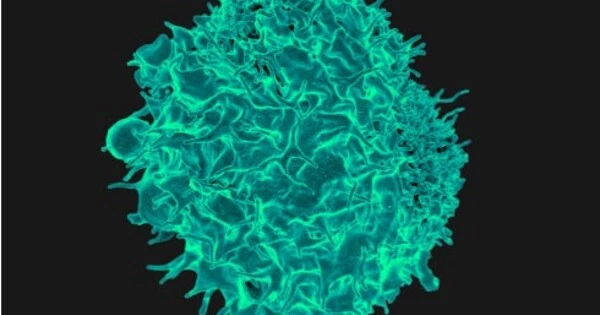
Immunological cell migration is a complex and dynamic process that is critical to the body’s immunological response. While immune cells were long assumed to travel predominantly by passive circulation through the bloodstream, it is now known that they move independently and actively throughout tissues. This discovery has contributed to a better understanding of immune cell behavior and their ability to navigate varied microenvironments in order to carry out their activities.
Human immune cells are more capable of controlling their own movement than previously assumed. Jonna Alanko, an InFLAMES researcher, showed that immune cells do not just follow chemical cues in their environment. On the contrary, they can alter these cues and self-organize their navigation in complex settings.
Cell migration in one direction is a necessary and fundamental phenomenon of life. It is a necessary condition for individual growth, blood vessel reconstruction, and immune response, among other things.
When immune cells are capable of creating chemokine gradients, they can avoid upcoming obstacles in complex environments and guide their own directional movement as well as that of other immune cells.
Jonna Alanko
Jonna Alanko, a postdoctoral researcher, conducted research on the migration and navigation of immune cells within the body. Chemokines, a type of signaling protein, serve an important function in directing immune cells to specific sites. Chemokines are generated in the lymph nodes, for example, and provide chemical cues termed chemokine gradients for cells to follow throughout the body. These chemokine gradients, according to Alanko, are like a fragrance trail in the air that grows lighter as you travel further away from its source.
The conventional wisdom holds that immune cells recognize their targets by following chemokine gradients. In other words, the cells that respond to these stimuli have been viewed as passive actors, which is not the truth.
“We were able to prove for the first time that contrary to the previous conception, immune cells do not need an existing chemokine gradient to find their way. They can create gradients themselves and thereby migrate collectively and efficiently even in complex environments,” explains Alanko.
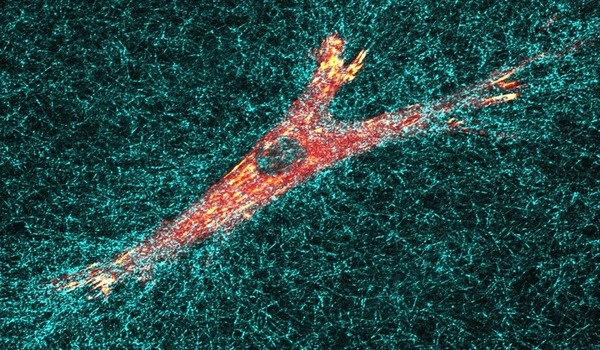
Cells consume chemokines
Immune cells have receptors that enable them to detect chemokine signals. CCR7 is one of these receptors, and it is located in dendritic cells. Dendritic cells are antigen-presenting cells that play a key role in initiating the complete immune response. They must find an infection, recognize it, and then travel to the lymph nodes with the information. Dendritic cells in the lymph nodes collaborate with other immune system cells to initiate an immune response to infections.
Alanko’s research found that dendritic cells not only register chemokine signals via their CCR7 receptor, but they also actively change their chemical environment by eating chemokines. By doing so, the cells generate local gradients that direct their own migration as well as the movement of other immune cells. T-cells, another type of immune cell, can also benefit from these self-generated gradients to improve their own directional movement, according to the researchers.
“When immune cells are capable of creating chemokine gradients, they can avoid upcoming obstacles in complex environments and guide their own directional movement as well as that of other immune cells,” Jonna Alanko adds.
This research adds to our understanding of how immune responses within the body are coordinated. It can, however, disclose how cancer cells direct their travel to form metastases.
“The CCR7 receptor has also been discovered in many cancer types, and the receptor has been shown to promote cancer metastasis in these cases.” Cancer cells may even employ the same mechanism to guide their migration as immune cells. As a result, Jonna Alanko believes that her findings “may help design new strategies to modify immune responses as well as target specific cancers.”