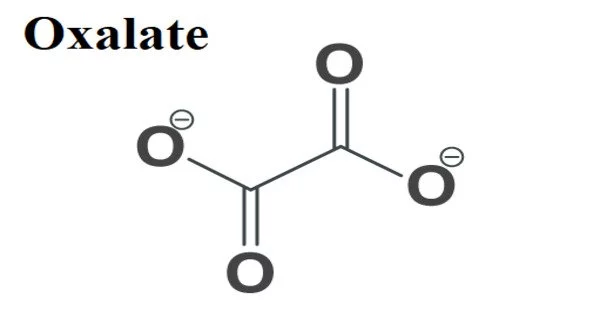
Oxalate is an anion having the molecular formula C2O42-. This dianion has no color. It is a naturally occurring molecule in plants and is also known as oxalic acid. These plant-based oxalates are both ingested and generated as waste by our bodies. The majority of plant-based foods contain oxalates, but they are also high in a variety of vital vitamins and minerals. It functions as both a human and a plant metabolite. It is a dicarboxylic acid dianion and an oxalate.
It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate [C2O4(CH3)2]. It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.
Properties
It is found in many plants and is produced by the incomplete oxidation of carbohydrates. Buckwheat and rhubarb roots and leaves are two examples of oxalate-rich plants. It contributes via being a plant metabolite or a human metabolite.
- Chemical formula: C2O42-
- Molar mass: 88.019 g·mol−1
- Conjugate acid: Hydrogenoxalate
- No. of hydrogen bond acceptor: 4
- Monoisotopic mass: 87.98 g/mol
- No. of hydrogen bond donor: 0
Structure
Simple oxalate salts show as a planar conformation with D2h molecular symmetry and as a conformation where the O-C-C-O dihedrals approach at a 90° angle with an approximate D2d symmetry in X-ray crystallography.
The oxalate anion exists in a nonplanar shape with approaching D2d symmetry where the O-C-C-O dihedrals approach 90°. Oxalate takes on the planar, D2h structure when chelated to cations. The O-C-C-O dihedral angle in Cs2C2O4 structure, on the other hand, is 81(1)°. As a result of the staggered nature of the two CO2 planes, Cs2C2O4 is more closely approximated by a D2d symmetry structure. Single-crystal X-ray diffraction identified two structural variants of Rb2C2O4: one with a planar oxalate and the other with a staggered oxalate.
Occurrence in nature
Oxalate is found in many plants and is produced through the partial oxidation of carbohydrates. Several plant foods, including spinach root and/or leaves, rhubarb, and buckwheat, are high in oxalic acid and may lead to the production of kidney stones in some people. Fat hen (“lamb’s quarters”), sorrel, and other Oxalis species are also oxalate-rich plants. Oxalic acid is abundant in the roots and/or leaves of rhubarb and buckwheat.
Other edible plants with significant concentrations of oxalate include, in decreasing order, star fruit (carambola), black pepper, parsley, poppy seed, amaranth, chard, beets, cocoa, chocolate, most nuts, most berries, fishtail palms, New Zealand spinach (Tetragonia tetragonioides), and beans.
Uses
- Oxalate such as calcium oxalate is used in the manufacturing of ceramic glazes.
- Escitalopram Oxalate is used in the treatment of anxiety and depression.
- In its powdered form, it is used as a pesticide in beekeeping.
- It acts as an excellent ligand for metal ions.
Health Hazards
In the human body, oxalic acid interacts with divalent metallic cations like iron (II) and calcium to generate crystals of the corresponding oxalates. These crystals are then expelled as minute crystals in urine. These oxalates can combine to form larger kidney stones, which can clog the kidney tubules. Calcium oxalate is responsible for around 80% of kidney stone formation.