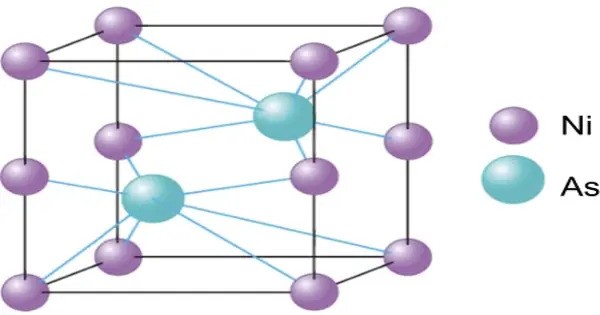
Nickel arsenide is a compound of nickel and arsenic and component of the ore nickeline. It is a binary compound composed of nickel (Ni) and arsenic (As) elements, typically found in the stoichiometric ratio of 1:1. It is a type of intermetallic compound, with nickel and arsenic atoms arranged in a crystal structure that is known for its high symmetry and metallic properties.
Due to its metallic properties and the presence of nickel, NiAs has potential applications in high-temperature materials, electronics, and catalysis. However, its primary uses are often limited due to the toxicity of arsenic. It is highly toxic and a known carcinogen in humans. Uncontrolled decomposition of nickel arsenide can give rise to further toxic nickel compounds.
Preparation of NiAs
The main compound within nickeline, nickel arsenide (NiAs), can be prepared by direct combination of the elements:
Ni(s) + As(s) → NiAs(s)
NiAs can be synthesized through a variety of methods, such as high-temperature reactions between nickel and arsenic, typically at temperatures of around 500–800°C.
Properties
It can be metallic gray or silvery in appearance. It is typically a poor conductor of electricity compared to pure metals like nickel.
- Chemical formula: AsNi
- Molar mass: 133.6150 g·mol−1
- Appearance: red solid
- Density: 7.57 g/cm3
- Melting point: 968 °C (1,774 °F; 1,241 K)
- Solubility in water: nearly insoluble
Reactivity
As a compound containing arsenic, nickel arsenide may be toxic if inhaled or ingested, particularly due to the toxicity of arsenic. It can react with acids, producing arsine gas (AsH₃), which is highly toxic. It may also be reactive with oxygen at high temperatures, forming nickel oxide and arsenic oxides.
Natural Occurrence
Nickel arsenide occurs naturally as a mineral known as nickeline or white nickel. This mineral is found in certain nickel-rich deposits, typically in association with other arsenic-containing minerals like cobalt arsenides.
The mineral forms as metallic ores in hydrothermal and magmatic environments.