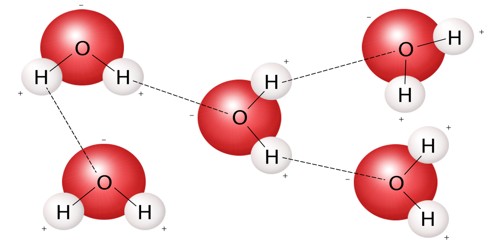
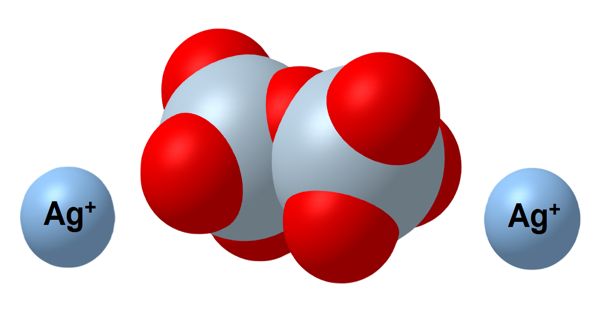
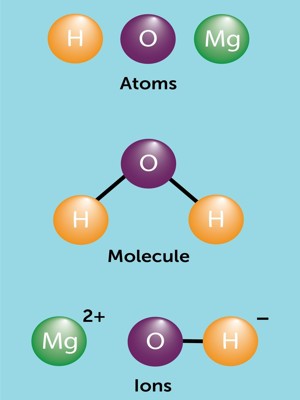
A molecule is the smallest amount of a chemical substance that can exist. It is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. If a molecule were split into smaller pieces, it would be a different substance. Examples of such elements are oxygen and chlorine.
Molecules are made up of atoms that are stuck together in a particular shape or form. The atoms of some elements do not easily bond with other atoms. Molecules form when two or more atoms form chemical bonds with each other. Not all combinations of atoms are equally possible; atoms make certain shapes in preference to others. Also, they have different valency. For example, oxygen atoms always have two bonds with other atoms, carbon atoms always have four bonds with other atoms, and nitrogen atoms always have three bonds with other atoms. Carbon dioxide is a molecule made of two oxygen atoms bonded to a carbon atom. It contains three atoms and is called a triatomic molecule.
TYPES –
Diatomic Molecules: A diatomic atom is composed of only two atoms, of the same or different chemical elements. Examples of diatomic molecules are O2 and CO.
Heteronuclear Diatomic Molecules: A heteronuclear diatomic molecule consists of two atoms of the same elements combined. There are seven diatomic elements: Hydrogen (H2), Nitrogen (N2), Oxygen ( O2), Fluorine ( (F2), Chlorine ( (Cl2), –Iodine (I2) and Bromine (Br2).
A molecule is formed when two or more atoms of an element chemically join together. In gases like air, the molecules are just flying around. In liquids like water, the molecules are stuck together but they can still move. If the types of atoms are different from each other, a compound is formed. In solids like sugar, the molecules can only vibrate. In the fourth state of matter known as plasma, the atoms are ionized and cannot form molecules.
Molecular size varies depending on the number of atoms that make up the molecule. With a molecular formula, you can write down the numbers of all atoms in a molecule. For example, the molecular formula of glucose is C6H12O6. That means that one molecule of glucose is made up of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.