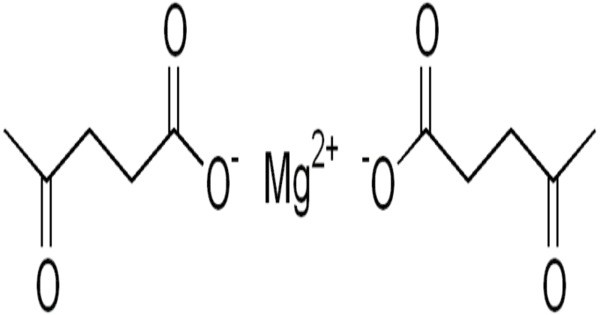
Magnesium levulinate, the magnesium salt of levulinic acid, is a mineral supplement. It is a magnesium salt of levulinic acid (also known as 4-oxopentanoic acid). It’s not widely used or well-known like magnesium citrate or magnesium sulfate,
Preparation
Magnesium levulinate is typically synthesized by neutralizing levulinic acid with a magnesium source like magnesium oxide (MgO) or magnesium hydroxide (Mg(OH)₂) in an aqueous medium:
2 C5H8O3 (levulinic acid) + Mg(OH)2 → Mg(C5H7O3)2 + 2 H2O
Properties
- Molar mass: ~254.52 g/mol
- Structure: It consists of two levulinate (4-oxopentanoate) anions coordinated with a single magnesium ion (Mg²⁺).
- Appearance: Typically appears as a white to off-white powder or crystalline solid.
- Solubility: Soluble in water, as magnesium salts generally are.
- Stability: Stable under normal conditions, but sensitive to moisture and should be stored in a dry environment.
- Reactivity: Reacts as a mild base, typical of magnesium salts. Not particularly reactive with most substances.
Natural Occurrence
Magnesium levulinate doesn’t occur naturally in significant quantities. Levulinic acid, its precursor, can be found as a byproduct in biomass decomposition, especially during the processing of cellulose under acidic conditions.
Applications
Pharmaceutical/Cosmetic Industry:
- May be used as a magnesium supplement in formulations.
- Potential use in dermatological products for its skin-friendly properties.
- Levulinate esters and salts have antimicrobial properties, which may enhance topical product preservation.
Chemical Industry/Biotechnology:
- Can serve as an intermediate in green chemistry or bio-based production routes.
- Levulinic acid and its derivatives are considered important platform chemicals for sustainable production.