Lutetium aluminum garnet is a compound or alloy made up of the elements lutetium (Lu) and aluminum (Al). LuAG (molecular formula Lu3Al5O12) is an inorganic chemical with a distinctive crystal structure well known for its application in high-efficiency laser systems.
LuAG is also beneficial in the synthesis of translucent ceramics. Lutetium has the symbol Lu and the atomic number 71, while aluminum has the sign Al and the atomic number 13. Lutetium is a rare earth metal, and aluminum is a common metal.
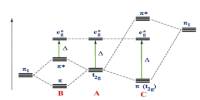
LuAG is a dopable scintillating crystal that emits light after being excited. Scintillating crystals are chosen for their great structural perfection, density, and effective atomic number. Because of its high density and thermal conductivity, LuAG is preferred over other crystals.
In comparison to other rare-earth garnets, LuAG has a comparatively small lattice constant, resulting in a higher density and a crystal field with smaller line widths and stronger energy level splitting in absorption and emission. These qualities make it a suitable host for active ions used in diode-pumped solid-state lasers such as Yb, Tm, Er, and Ho.
Properties
- Chemical Composition: LuAG is composed of lutetium (Lu3+), aluminum (Al3+), and oxygen (O2-). Its chemical formula is Lu3Al5O12.
- Crystal Structure: LuAG typically crystallizes in the cubic system, with a crystal lattice structure that forms a three-dimensional network. The cubic structure contributes to its optical transparency and scintillation properties.
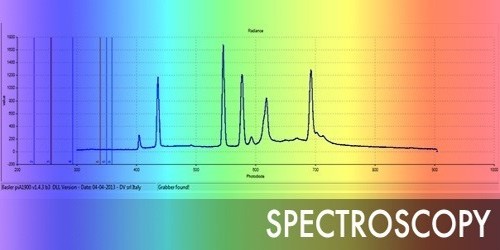
- Scintillation Properties: LuAG is often used as a scintillator, a material that emits visible or ultraviolet light when ionizing radiation interacts with it. This property makes it valuable in various applications, including medical imaging, particle detectors, and gamma-ray spectroscopy.
- Optical Properties: It is transparent throughout a wide wavelength range, making it appropriate for a variety of optical applications. It’s employed in high-energy physics investigations, laser technology, and as a platform for other materials’ epitaxial development.
- Rare Earth Element: Because lutetium (Lu) is a rare earth element, its rarity makes LuAG relatively expensive. To improve the scintillation capabilities of LuAG crystals, lutetium is frequently employed as a dopant.
- Growth Methods: The Czochralski process, the floating zone approach, and the Bridgman-Stockbarger method are commonly used to create LuAG crystals. These techniques enable the controlled development of high-quality crystals.
Applications
LuAG has a variety of applications, including use in positron emission tomography (PET) scanners, gamma-ray detectors, and high-energy physics experiments. Its high density and radiation resistance make it suitable for environments where radiation is a concern.
The density of the lutetium crystal is greater than that of other metals, such as yttrium, indicating that the crystal characteristics do not alter with the addition of dopant ions. Because of its density and thermal durability, it can be very beneficial for high-energy particle detection and quantification. Despite its superior physical qualities, this crystal is less often employed than its brother garnets due to its high melting temperature and lack of lutetium availability.